A randomized phase III study comparing pegylated liposomal doxorubicin with capecitabine as first-line chemotherapy in elderly patients with metastatic breast cancer: results of the OMEGA study of the Dutch Breast Cancer Research Group BOOG
- PMID: 24504445
- PMCID: PMC4433520
- DOI: 10.1093/annonc/mdt588
A randomized phase III study comparing pegylated liposomal doxorubicin with capecitabine as first-line chemotherapy in elderly patients with metastatic breast cancer: results of the OMEGA study of the Dutch Breast Cancer Research Group BOOG
Abstract
Background: Prospective data on chemotherapy for elderly patients with metastatic breast cancer (MBC) remain scarce. We compared the efficacy and safety of first-line chemotherapy with pegylated liposomal doxorubicin (PLD) versus capecitabine in MBC patients aged ≥65 years in a multicentre, phase III trial.
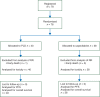
Patients and methods: Patients were randomized to six cycles of PLD (45 mg/m(2) every 4 weeks) or eight cycles of capecitabine (1000 mg/m(2) twice daily, day 1-14 every 3 weeks).
Results: The study enrolled 78 of the planned 154 patients and was closed prematurely due to slow accrual and supply problems of PLD. Many included patients were aged ≥75 years (54%) and vulnerable (≥1 geriatric condition: 71%). The median dose intensity was 85% for PLD and 84% for capecitabine, respectively. In both arms, the majority of patients completed at least 12 weeks of treatment (PLD 73%; capecitabine 74%). After a median follow-up of 39 months, 77 patients had progressed and 62 patients had died of MBC. Median progression-free survival was 5.6 versus 7.7 months (P = 0.11) for PLD and capecitabine, respectively. Median overall survival was 13.8 months for PLD and 16.8 months for capecitabine (P = 0.59). Both treatments were feasible, grade 3 toxicities consisting of fatigue (both arms: 13%), hand-foot syndrome (PLD: 10%; capecitabine: 16%), stomatitis (PLD: 10%; capecitabine: 3%), exanthema (PLD: 5%) and diarrhoea (PLD: 3%; capecitabine: 5%). Only 1 of 10 patients aged ≥80 years completed chemotherapy, while 3 and 6 patients discontinued treatment due to toxicity or progressive disease, respectively.
Conclusion: Both PLD and capecitabine demonstrated comparable efficacy and acceptable tolerance as first-line single-agent chemotherapy in elderly patients with MBC, even in vulnerable patients or patients aged ≥75 years. However, patients aged ≥80 years were unlikely to complete chemotherapy successfully.
Clinical trial numbers: EudraCT 2006-002046-10; ISRCTN 11114726; CKTO 2006-09; BOOG 2006-02.
Keywords: capecitabine; elderly; geriatric; metastatic breast cancer; pegylated liposomal doxorubicin; phase III.
Figures
References
-
- Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer. Breast. 2012;21:242–252. - PubMed
-
- Debled M, Bellera C, Donamaria C, et al. Chemotherapy treatment for older women with metastatic breast cancer: what is the evidence? Cancer Treat Rev. 2011;37:590–598. - PubMed
-
- O'Shaughnessy JA, Blum J, Moiseyenko J, et al. Randomized, open label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate, 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol. 2001;12:1247–1254. - PubMed
-
- Feher O, Vodvarka P, Jassem J, et al. First-line gemcitabine versus epirubicin in postmenopausal women aged 60 or older with metastatic breast cancer: a multicenter, randomized, phase III study. Ann Oncol. 2005;6:899–908. - PubMed
-
- Hamberg P, Verweij J, Seyanaeve C. Cytotoxic therapy for the elderly with metastatic breast cancer: a review on safety, pharmacokinetics and efficacy. Eur J Cancer. 2007;43:1514–1528. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical