MUC1-C nuclear localization drives invasiveness of renal cancer cells through a sheddase/gamma secretase dependent pathway
- PMID: 24504508
- PMCID: PMC3996672
- DOI: 10.18632/oncotarget.1768
MUC1-C nuclear localization drives invasiveness of renal cancer cells through a sheddase/gamma secretase dependent pathway
Abstract
MUC1 is a membrane-anchored mucin and its cytoplasmic tail (CT) can interact with many signaling pathways and act as a co-transcription factor to activate genes involved in tumor progression and metastasis. MUC1 is overexpressed in renal cell carcinoma with correlation to prognosis and has been implicated in the hypoxic pathway, the main renal carcinogenetic pathway. In this context, we assessed the effects of MUC1 overexpression on renal cancer cells properties. Using shRNA strategy and/or different MUC1 constructs, we found that MUC1-extracellular domain and MUC1-CT are involved in increase of migration, cell viability, resistance to anoikis and in decrease of cell aggregation in cancer cells. Invasiveness depends only on MUC1-CT. Then, by using siRNA strategy and/or pharmacological inhibitors or peptides, we showed that sheddases ADAM10, ADAM17 and gamma-secretase are necessary for MUC1 C-terminal subunit (MUC1-C) nuclear location and in increase of invasion property. Finally, MUC1 overexpression increases ADAM10/17 protein expression suggesting a positive regulatory loop. In conclusion, we report that MUC1 acts in renal cancer progression and MUC1-C nuclear localization drives invasiveness of cancer cells through a sheddase/gamma secretase dependent pathway. MUC1 appears as a therapeutic target by blocking MUC1 cleavage or nuclear translocation by using pharmacological approach and peptide strategies.
Figures

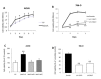
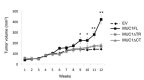
 ), with MUC1FL- (
), with MUC1FL- ( ), with MUC1ΔTR- (
), with MUC1ΔTR- ( ) or with MUC1ΔCT (
) or with MUC1ΔCT ( )-expression vectors were performed in SCID mice. Values are means s.e.m and represent values obtained in 6 mice (* p<0.05, ** p<0.01).
)-expression vectors were performed in SCID mice. Values are means s.e.m and represent values obtained in 6 mice (* p<0.05, ** p<0.01).

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

