Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection
- PMID: 24504509
- PMCID: PMC3913641
- DOI: 10.1371/journal.pone.0087572
Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection
Abstract
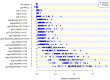
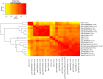
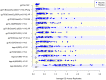
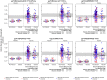
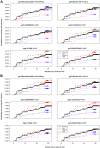
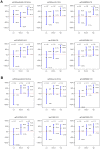
In the RV144 HIV-1 vaccine efficacy trial, IgG antibody (Ab) binding levels to variable regions 1 and 2 (V1V2) of the HIV-1 envelope glycoprotein gp120 were an inverse correlate of risk of HIV-1 infection. To determine if V1V2-specific Abs cross-react with V1V2 from different HIV-1 subtypes, if the nature of the V1V2 antigen used to asses cross-reactivity influenced infection risk, and to identify immune assays for upcoming HIV-1 vaccine efficacy trials, new V1V2-scaffold antigens were designed and tested. Protein scaffold antigens carrying the V1V2 regions from HIV-1 subtypes A, B, C, D or CRF01_AE were assayed in pilot studies, and six were selected to assess cross-reactive Abs in the plasma from the original RV144 case-control cohort (41 infected vaccinees, 205 frequency-matched uninfected vaccinees, and 40 placebo recipients) using ELISA and a binding Ab multiplex assay. IgG levels to these antigens were assessed as correlates of risk in vaccine recipients using weighted logistic regression models. Levels of Abs reactive with subtype A, B, C and CRF01_AE V1V2-scaffold antigens were all significant inverse correlates of risk (p-values of 0.0008-0.05; estimated odds ratios of 0.53-0.68 per 1 standard deviation increase). Thus, levels of vaccine-induced IgG Abs recognizing V1V2 regions from multiple HIV-1 subtypes, and presented on different scaffolds, constitute inverse correlates of risk for HIV-1 infection in the RV144 vaccine trial. The V1V2 antigens provide a link between RV144 and upcoming HIV-1 vaccine trials, and identify reagents and methods for evaluating V1V2 Abs as possible correlates of protection against HIV-1 infection.
Trial registration: ClinicalTrials.gov NCT00223080.
Conflict of interest statement
Figures



References
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, et al. (2009) Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N Engl J Med 361: 2209–2220. - PubMed
-
- Pinter A, Honnen WJ, Kayman SC, Trochev O, Wu Z (1998) Potent neutralization of primary HIV-1 isolates by antibodies directed against epitopes present in the V1/V2 domain of HIV-1 gp120. Vaccine 16: 1803–1811. - PubMed
-
- Gorny MK, Xu J-Y, Karwowska S, Buchbinder A, Zolla-Pazner S (1993) Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol 150: 635–643. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

