Occlusion for stimulus deprivation amblyopia
- PMID: 24504975
- PMCID: PMC4260153
- DOI: 10.1002/14651858.CD005136.pub3
Occlusion for stimulus deprivation amblyopia
Update in
-
Occlusion for stimulus deprivation amblyopia.Cochrane Database Syst Rev. 2020 Mar 23;3(3):CD005136. doi: 10.1002/14651858.CD005136.pub4. Cochrane Database Syst Rev. 2020. PMID: 32203629 Free PMC article.
Abstract
Background: Stimulus deprivation amblyopia (SDA) develops due to an obstruction to the passage of light secondary to a condition such as cataract. The obstruction prevents formation of a clear image on the retina. SDA can be resistant to treatment, leading to poor visual prognosis. SDA probably constitutes less than 3% of all amblyopia cases, although precise estimates of prevalence are unknown. In developed countries, most patients present under the age of one year; in less developed parts of the world patients are likely to be older at the time of presentation. The mainstay of treatment is removal of the cataract and then occlusion of the better-seeing eye, but regimens vary, can be difficult to execute, and traditionally are believed to lead to disappointing results.
Objectives: Our objective was to evaluate the effectiveness of occlusion therapy for SDA in an attempt to establish realistic treatment outcomes. Where data were available, we also planned to examine evidence of any dose response effect and to assess the effect of the duration, severity, and causative factor on the size and direction of the treatment effect.
Search methods: We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library 2013, Issue 9), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to October 2013), EMBASE (January 1980 to October 2013), the Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to October 2013), PubMed (January 1946 to October 2013), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com ), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 28 October 2013.
Selection criteria: We planned to include randomized and quasi-randomized controlled trials of participants with unilateral SDA with visual acuity worse than 0.2 LogMAR or equivalent. We did not specify any restrictions for inclusion based upon age, gender, ethnicity, co-morbidities, medication use, or the number of participants.
Data collection and analysis: Two review authors independently assessed study abstracts identified by the electronic searches.
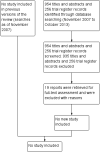
Main results: We did not identify any trials that met the inclusion criteria specified in the protocol for this review.
Authors' conclusions: We found no evidence on the effectiveness of any treatment for SDA. Future randomized controlled trials are needed in order to evaluate the safety and effectiveness of occlusion, duration of treatment, level of vision that can be realistically achieved, effects of age at onset and magnitude of visual defect, optimum occlusion regimen, and factors associated with satisfactory and unsatisfactory outcomes with the use of various interventions for SDA.
Figures
Update of
-
Interventions for stimulus deprivation amblyopia.Cochrane Database Syst Rev. 2006 Jul 19;(3):CD005136. doi: 10.1002/14651858.CD005136.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2014 Feb 06;(2):CD005136. doi: 10.1002/14651858.CD005136.pub3. PMID: 16856079 Free PMC article. Updated.
References
-
- Agafonov VV. Development of a technique for ametropia correction with phakic intraocular lenses. Vestnik Rossiiskoi akademii meditsinskikh nauk / Rossiiskaia akademiia meditsinskikh nauk. 2007;(8):52–6. - PubMed
-
- Arruga A. Occlusion therapy in children. International Ophthalmology Clinics. 1966;6(3):435–52. - PubMed
-
- Botabekova TK, Kurgambekova NS. A comparative analysis of the efficiency of different methods in the treatment of amblyopia. Vestnik Oftalmologii. 2004;120(5):40–1. - PubMed
-
- Cramer FE, Lamela N, Luqui Lagleyze J, Corsellas A. Use of the electronic flash in the pleoptic treatment of amblyopias [Uso del flash electronico en el tratamiento pleoptico de las ambliopias]. Arquivos Brasileiros de Oftalmologia. 1966;29(3):83–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous