Involvement of angiopoietin-like 4 in matrix remodeling during chondrogenic differentiation of mesenchymal stem cells
- PMID: 24505142
- PMCID: PMC3961665
- DOI: 10.1074/jbc.M113.539825
Involvement of angiopoietin-like 4 in matrix remodeling during chondrogenic differentiation of mesenchymal stem cells
Abstract
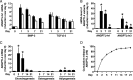
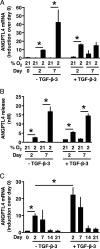
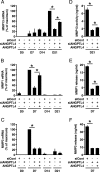
Mesenchymal stem cells (MSCs) are considered for cartilage engineering given their ability to differentiate into chondrocytes. Chondrogenic differentiation of MSCs is currently triggered by micromass culture in the presence of a member of the TGF-β superfamily. However, the main constituents of the cartilaginous matrix, aggrecan and type II collagen, are degraded at the end of the differentiation process through induction of matrix metallopeptidase (MMP)13. We hypothesized that MSCs undergoing chondrogenic differentiation produce an intermediate cytokine that triggers this matrix remodeling. Analysis of transcriptomic data identified angiopoietin-like 4 (ANGPTL4) as one of the most strongly up-regulated gene encoding a secreted factor during TGF-β-induced chondrogenesis. To gain insight into the role of ANGPTL4 during chondrogenesis, we used recombinant ANGPTL4 as well as a RNA interference approach. Addition of exogenous ANGPTL4 during the course of TGF-β-induced differentiation reduced the mRNA levels of aggrecan and type II collagen, although it increased those of MMP1 and MMP13. Accordingly, deposition of aggrecan and total collagens was diminished, whereas release of MMP1 and MMP13 was increased. Conversely, transfection of MSCs with an siRNA targeting ANGPTL4 prior to induction of chondrogenesis increased expression of type II collagen and aggrecan, whereas it repressed that of MMP1, MMP3, and MMP13. A neutralizing antibody against integrin αVβ5, a known receptor for ANGPTL4, mimicked some of the effects observed after siRNA-mediated ANGPTL4 silencing. Our data provide evidence that ANGPTL4 promotes cartilage matrix remodeling by inhibiting expression of its two key components and by up-regulating the level of certain MMPs.
Keywords: Cartilage; Cell Differentiation; Chondrocytes; Chondrogenesis; Extracellular Matrix Proteins; Integrin; Matrix Metalloproteinase (MMP); Mesenchymal Stem Cells; Tissue Engineering; Transforming Growth Factor Beta (TGFbeta).
Figures





References
-
- Burrage P. S., Mix K. S., Brinckerhoff C. E. (2006) Matrix metalloproteinases: role in arthritis. Front Biosci. 11, 529–543 - PubMed
-
- Jorgensen C., Noël D. (2011) Mesenchymal stem cells in osteoarticular diseases. Regen. Med. 6, 44–51 - PubMed
-
- Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S., Marshak D. R. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 - PubMed
-
- Poole A. R., Kojima T., Yasuda T., Mwale F., Kobayashi M., Laverty S. (2001) Composition and structure of articular cartilage: a template for tissue repair. Clin. Orthop. Relat. Res. 391, S26–33 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

