Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system)
- PMID: 24505239
- PMCID: PMC3915094
- DOI: 10.7150/thno.7819
Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system)
Abstract
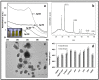
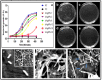
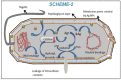
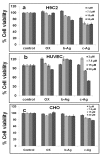
In this report, we have designed a simple and efficient green chemistry approach for the synthesis of colloidal silver nanoparticles (b-AgNPs) that is formed by the reduction of silver nitrate (AgNO3) solution using Olax scandens leaf extract. The colloidal b-AgNPs, characterized by various physico-chemical techniques exhibit multifunctional biological activities (4-in-1 system). Firstly, bio-synthesized silver nanoparticles (b-AgNPs) shows enhanced antibacterial activity compared to chemically synthesize silver nanoparticles (c-AgNPs). Secondly, b-AgNPs show anti-cancer activities to different cancer cells (A549: human lung cancer cell lines, B16: mouse melanoma cell line & MCF7: human breast cancer cells) (anti-cancer). Thirdly, these nanoparticles are biocompatible to rat cardiomyoblast normal cell line (H9C2), human umbilical vein endothelial cells (HUVEC) and Chinese hamster ovary cells (CHO) which indicates the future application of b-AgNPs as drug delivery vehicle. Finally, the bio-synthesized AgNPs show bright red fluorescence inside the cells that could be utilized to detect the localization of drug molecules inside the cancer cells (a diagnostic approach). All results together demonstrate the multifunctional biological activities of bio-synthesized AgNPs (4-in-1 system) that could be applied as (i) anti-bacterial & (ii) anti-cancer agent, (iii) drug delivery vehicle, and (iv) imaging facilitator. To the best of our knowledge, there is not a single report of biosynthesized AgNPs that demonstrates the versatile applications (4-in-1 system) towards various biomedical applications. Additionally, a plausible mechanistic approach has been explored for the synthesis of b-AgNPs and its anti-bacterial as well as anti-cancer activity. We strongly believe that bio-synthesized AgNPs will open a new direction towards various biomedical applications in near future.
Keywords: Antibacterial; Bio-synthesis; Green Chemistry; Multifunctional activities; Olax scandens; Silver nanoparticle; anti-cancer..
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures









References
-
- Alivisatos P. The use of nanocrystals in biological detection. Nature Biotechnology. 2004;22:47–52. - PubMed
-
- Gao X, Cui Y, Levenson RM, Chung LWK, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotechnology. 2004;22:969–76. - PubMed
-
- Patra CR, Bhattacharya R, Patra S, Vlahakis NE, Gabashvili A, Koltypin Y. et al. Pro-angiogenic properties of europium(III) hydroxide nanorods. Advanced Materials. 2008;20(4):753–6.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

