[¹⁸F]FDG positron emission tomography within two weeks of starting erlotinib therapy can predict response in non-small cell lung cancer patients
- PMID: 24505298
- PMCID: PMC3914822
- DOI: 10.1371/journal.pone.0087629
[¹⁸F]FDG positron emission tomography within two weeks of starting erlotinib therapy can predict response in non-small cell lung cancer patients
Abstract
Purpose: The aim of this prospective study was to evaluate whether [¹⁸F]FDG-PET/CT, performed within two weeks of starting erlotinib therapy can predict tumor response defined by RECIST 1.1 criteria after 8 weeks of treatment in patients with inoperable (stage IIIA to IV) non-small cell lung cancer patients.
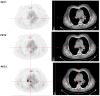
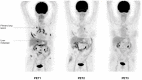
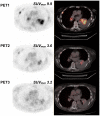
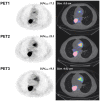
Patients and methods: Three [¹⁸F]FDG-PET/CT scans were acquired in 12 patients before (5±4 days) and after 9±3 days (early PET) and 60±6 days (late PET) of erlotinib therapy. Conventional evaluation, including at least chest CT (baseline versus after 8 weeks of treatment), was performed according to RECIST 1.1 criteria. Change in [¹⁸F]FDG uptake was compared with conventional response, progression-free survival (PFS), and overall survival (OS).
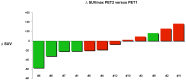
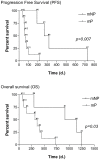
Results: By using ROC analysis, the Area Under the Curve for prediction of metabolic non-progressive disease (mNP) by early PET was 0.86 (95% CI, 0.62 to 1.1; P = 0.04) at a cut-off of 21.6% reduction in maximum Standardized Uptake Value (SUVmax). This correctly classified 11/12 patients (7 with true progressive disease; 4 with true non-progressive disease; 1 with false progressive disease). Non-progressive disease after 8 weeks of treatment according to RECIST 1.1 criteria was significantly more frequent in patients classified mNP (P = 0.01, Fisher's exact test). mNP patients showed prolonged PFS (HR = 0.27; 95% CI, 0.04 to 0.59; P<0.01) and OS (HR = 0.34; 95% CI, 0.06 to 0.84; P = 0.03). Late PET analysis provided concordant results.
Conclusion: Morphologic response, PFS and OS survival in non-small cell lung cancer patients can be predicted by [¹⁸F]FDG-PET/CT scan within 2 weeks after starting erlotinib therapy.
Conflict of interest statement
Figures

References
-
- Ferlay J, Parkin DM, Steliarova-Foucher E (2010) Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 46: 765–781. - PubMed
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. - PubMed
-
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, et al. (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346: 92–98. - PubMed
-
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, et al. (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350: 2129–2139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

