Role of docosahexaenoic acid treatment in improving liver histology in pediatric nonalcoholic fatty liver disease
- PMID: 24505350
- PMCID: PMC3913708
- DOI: 10.1371/journal.pone.0088005
Role of docosahexaenoic acid treatment in improving liver histology in pediatric nonalcoholic fatty liver disease
Abstract
Introduction: Nonalcoholic fatty liver disease (NAFLD) is one of the most important causes of liver-related morbidity and mortality in children. Recently, we have reported the effects of docosahexaenoic acid (DHA), the major dietary long-chain polyunsaturated fatty acids, in children with NAFLD. DHA exerts a potent anti-inflammatory activity through the G protein-coupled receptor (GPR)120. Our aim was to investigate in pediatric NAFLD the mechanisms underlying the effects of DHA administration on histo-pathological aspects, GPR120 expression, hepatic progenitor cell activation and macrophage pool.
Patients and methods: 20 children with untreated NAFLD were included. Children were treated with DHA for 18 months. Liver biopsies before and after the treatment were analyzed. Hepatic progenitor cell activation, macrophage pool and GPR120 expression were evaluated and correlated with clinical and histo-pathological parameters.
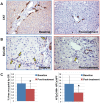
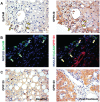
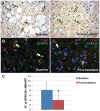
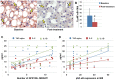
Results: GPR120 was expressed by hepatocytes, liver macrophages, and hepatic progenitor cells. After DHA treatment, the following modifications were present: i) the improvement of histo-pathological parameters such as NAFLD activity score, ballooning, and steatosis; ii) the reduction of hepatic progenitor cell activation in correlation with histo-pathological parameters; iii) the reduction of the number of inflammatory macrophages; iv) the increase of GPR120 expression in hepatocytes; v) the reduction of serine-311-phosphorylated nuclear factor kappa B (NF-κB) nuclear translocation in hepatocytes and macrophages in correlation with serum inflammatory cytokines.
Conclusions: DHA could modulate hepatic progenitor cell activation, hepatocyte survival and macrophage polarization through the interaction with GPR120 and NF-κB repression. In this scenario, the modulation of GPR120 exploits a novel crucial role in the regulation of the cell-to-cell cross-talk that drives inflammatory response, hepatic progenitor cell activation and hepatocyte survival.
Conflict of interest statement
Figures

References
-
- Alisi A, Feldstein AE, Villani A, Raponi M, Nobili V (2012) Pediatric nonalcoholic fatty liver disease: a multidisciplinary approach. Nat Rev Gastroenterol Hepatol 9: 152–161. - PubMed
-
- Nobili V, Carpino G, Alisi A, Franchitto A, Alpini G, et al. (2012) Hepatic progenitor cells activation, fibrosis and adipokines production in pediatric nonalcoholic fatty liver disease. Hepatology 56: 2142–2153. - PubMed
-
- Masterton GS, Plevris JN, Hayes PC (2010) Review article: omega-3 fatty acids - a promising novel therapy for non-alcoholic fatty liver disease. Aliment Pharmacol Ther 31: 679–692. - PubMed
-
- Parker HM, Johnson NA, Burdon CA, Cohn JS, O'Connor HT, et al. (2012) Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 56: 944–951. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

