Lycium barbarum polysaccharides prevent memory and neurogenesis impairments in scopolamine-treated rats
- PMID: 24505383
- PMCID: PMC3914900
- DOI: 10.1371/journal.pone.0088076
Lycium barbarum polysaccharides prevent memory and neurogenesis impairments in scopolamine-treated rats
Abstract
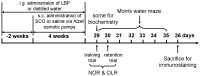
Lycium barbarum is used both as a food additive and as a medicinal herb in many countries, and L. barbarum polysaccharides (LBPs), a major cell component, are reported to have a wide range of beneficial effects including neuroprotection, anti-aging and anticancer properties, and immune modulation. The effects of LBPs on neuronal function, neurogenesis, and drug-induced learning and memory deficits have not been assessed. We report the therapeutic effects of LBPs on learning and memory and neurogenesis in scopolamine (SCO)-treated rats. LBPs were administered via gastric perfusion for 2 weeks before the onset of subcutaneous SCO treatment for a further 4 weeks. As expected, SCO impaired performance in novel object and object location recognition tasks, and Morris water maze. However, dual SCO- and LBP-treated rats spent significantly more time exploring the novel object or location in the recognition tasks and had significant shorter escape latency in the water maze. SCO administration led to a decrease in Ki67- or DCX-immunoreactive cells in the dentate gyrus and damage of dendritic development of the new neurons; LBP prevented these SCO-induced reductions in cell proliferation and neuroblast differentiation. LBP also protected SCO-induced loss of neuronal processes in DCX-immunoreactive neurons. Biochemical investigation indicated that LBP decreased the SCO-induced oxidative stress in hippocampus and reversed the ratio Bax/Bcl-2 that exhibited increase after SCO treatment. However, decrease of BDNF and increase of AChE induced by SCO showed no response to LBP administration. These results suggest that LBPs can prevent SCO-induced cognitive and memory deficits and reductions in cell proliferation and neuroblast differentiation. Suppression of oxidative stress and apoptosis may be involved in the above effects of LBPs that may be a promising candidate to restore memory functions and neurogenesis.
Conflict of interest statement
Figures









References
-
- Zhang M, Chen H, Huang J, Li Z, Zhu C, et al. (2005) Effect of lycium barbarum polysaccharide on human hepatoma QGY7703 cells: inhibition of proliferation and induction of apoptosis. Life Sci 76: 2115–2124. - PubMed
-
- Amagase H, Sun B, Borek C (2009) Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr Res 29: 19–25. - PubMed
-
- Gao XM, Xu ZM, Li ZW, editors (2000) Traditional Chinese Medicines. Beijing: Health Publishing House. 185 p.
-
- Li QY, editor (2001) Healthy Functions and Medicinal Prescriptions of Lycium barbarum (Gou Ji Zi). Beijing: Jindun Press. 1–205 p.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

