Identification and glycerol-induced correction of misfolding mutations in the X-linked mental retardation gene CASK
- PMID: 24505460
- PMCID: PMC3914952
- DOI: 10.1371/journal.pone.0088276
Identification and glycerol-induced correction of misfolding mutations in the X-linked mental retardation gene CASK
Abstract
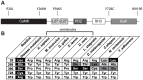
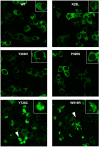
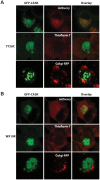
The overwhelming amount of available genomic sequence variation information demands a streamlined approach to examine known pathogenic mutations of any given protein. Here we seek to outline a strategy to easily classify pathogenic missense mutations that cause protein misfolding and are thus good candidates for chaperone-based therapeutic strategies, using previously identified mutations in the gene CASK. We applied a battery of bioinformatics algorithms designed to predict potential impact on protein structure to five pathogenic missense mutations in the protein CASK that have been shown to underlie pathologies ranging from X-linked mental retardation to autism spectrum disorder. A successful classification of the mutations as damaging was not consistently achieved despite the known pathogenicity. In addition to the bioinformatics analyses, we performed molecular modeling and phylogenetic comparisons. Finally, we developed a simple high-throughput imaging assay to measure the misfolding propensity of the CASK mutants in situ. Our data suggests that a phylogenetic analysis may be a robust method for predicting structurally damaging mutations in CASK. Mutations in two evolutionarily invariant residues (Y728C and W919R) exhibited a strong propensity to misfold and form visible aggregates in the cytosolic milieu. The remaining mutations (R28L, Y268H, and P396S) showed no evidence of aggregation and maintained their interactions with known CASK binding partners liprin-α3 Mint-1, and Veli, indicating an intact structure. Intriguingly, the protein aggregation caused by the Y728C and W919R mutations was reversed by treating the cells with a chemical chaperone (glycerol), providing a possible therapeutic strategy for treating structural mutations in CASK in the future.
Conflict of interest statement
Figures
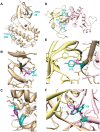

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

