Differential induction of TLR3-dependent innate immune signaling by closely related parasite species
- PMID: 24505488
- PMCID: PMC3914978
- DOI: 10.1371/journal.pone.0088398
Differential induction of TLR3-dependent innate immune signaling by closely related parasite species
Abstract
The closely related protozoan parasites Toxoplasma gondii and Neospora caninum display similar life cycles, subcellular ultrastructure, invasion mechanisms, metabolic pathways, and genome organization, but differ in their host range and disease pathogenesis. Type II (γ) interferon has long been known to be the major mediator of innate and adaptive immunity to Toxoplasma infection, but genome-wide expression profiling of infected host cells indicates that Neospora is a potent activator of the type I (α/β) interferon pathways typically associated with antiviral responses. Infection of macrophages from mice with targeted deletions in various innate sensing genes demonstrates that host responses to Neospora are dependent on the toll-like receptor Tlr3 and the adapter protein Trif. Consistent with this observation, RNA from Neospora elicits TLR3-dependent type I interferon responses when targeted to the host endo-lysosomal system. Although live Toxoplasma fail to induce type I interferon, heat-killed parasites do trigger this response, albeit much weaker than Neospora, and co-infection studies reveal that T. gondii actively suppresses the production of type I interferon. These findings reveal that eukaryotic pathogens can be potent inducers of type I interferon and that related parasite species interact with this pathway in distinct ways.
Conflict of interest statement
Figures

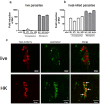

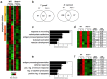

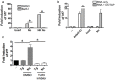
References
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, et al. (2013) Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382: 209–222. - PubMed
-
- Suzuki Y, Orellana MA, Schreiber RD, Remington JS (1988) Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240: 516–518. - PubMed
-
- Hayward AR, Chmura K, Cosyns M (2000) Interferon-gamma is required for innate immunity to Cryptosporidium parvum in mice. J Infect Dis 182: 1001–1004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32-AI75846/AI/NIAID NIH HHS/United States
- R01 AI042334/AI/NIAID NIH HHS/United States
- P30 DK050306/DK/NIDDK NIH HHS/United States
- R01 AI029646/AI/NIAID NIH HHS/United States
- R01-AI29646/AI/NIAID NIH HHS/United States
- R37 AI028724/AI/NIAID NIH HHS/United States
- R21 AI104998/AI/NIAID NIH HHS/United States
- R21-AI104998/AI/NIAID NIH HHS/United States
- R01-AI42334/AI/NIAID NIH HHS/United States
- P30-DK050306/DK/NIDDK NIH HHS/United States
- U19 AI083022/AI/NIAID NIH HHS/United States
- U19-AI083022/AI/NIAID NIH HHS/United States
- F32 AI075846/AI/NIAID NIH HHS/United States
- R37-AI28724/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

