Designing hydrolytic zinc metalloenzymes
- PMID: 24506795
- PMCID: PMC3985962
- DOI: 10.1021/bi4016617
Designing hydrolytic zinc metalloenzymes
Abstract
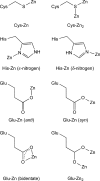
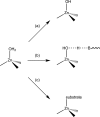
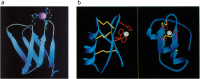
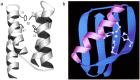
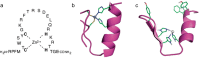
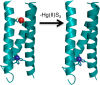
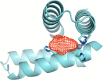
Zinc is an essential element required for the function of more than 300 enzymes spanning all classes. Despite years of dedicated study, questions regarding the connections between primary and secondary metal ligands and protein structure and function remain unanswered, despite numerous mechanistic, structural, biochemical, and synthetic model studies. Protein design is a powerful strategy for reproducing native metal sites that may be applied to answering some of these questions and subsequently generating novel zinc enzymes. From examination of the earliest design studies introducing simple Zn(II)-binding sites into de novo and natural protein scaffolds to current studies involving the preparation of efficient hydrolytic zinc sites, it is increasingly likely that protein design will achieve reaction rates previously thought possible only for native enzymes. This Current Topic will review the design and redesign of Zn(II)-binding sites in de novo-designed proteins and native protein scaffolds toward the preparation of catalytic hydrolytic sites. After discussing the preparation of Zn(II)-binding sites in various scaffolds, we will describe relevant examples for reengineering existing zinc sites to generate new or altered catalytic activities. Then, we will describe our work on the preparation of a de novo-designed hydrolytic zinc site in detail and present comparisons to related designed zinc sites. Collectively, these studies demonstrate the significant progress being made toward building zinc metalloenzymes from the bottom up.
Figures






References
-
- Andreini C.; Banci L.; Bertini I.; Rosato A. (2006) Counting the Zinc-Proteins Encoded in the Human Genome. J. Proteome Res. 5, 196–201. - PubMed
-
- Sousa S. F.; Lopes A. B.; Fernandes P. A.; Ramos M. J. (2009) The Zinc Proteome: A Tale of Stability and Functionality. Dalton Trans. 7946–7956. - PubMed
-
- Keilin D.; Mann T. (1939) Carbonic Anhydrase. Nature 144, 442–443.
-
- Vallee B. L.; Neurath H. (1954) Carboxypeptidase, a Zinc Metalloprotein. J. Am. Chem. Soc. 76, 5006–5007.
-
- Vallee B. L. (1959) Biochemistry, Physiology and Pathology of Zinc. Physiol. Rev. 39, 443–490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

