Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations
- PMID: 24507191
- PMCID: PMC3939050
- DOI: 10.1016/j.neuron.2013.12.018
Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations
Abstract
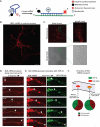
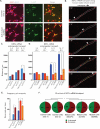
The RNA-binding protein TDP-43 regulates RNA metabolism at multiple levels, including transcription, RNA splicing, and mRNA stability. TDP-43 is a major component of the cytoplasmic inclusions characteristic of amyotrophic lateral sclerosis and some types of frontotemporal lobar degeneration. The importance of TDP-43 in disease is underscored by the fact that dominant missense mutations are sufficient to cause disease, although the role of TDP-43 in pathogenesis is unknown. Here we show that TDP-43 forms cytoplasmic mRNP granules that undergo bidirectional, microtubule-dependent transport in neurons in vitro and in vivo and facilitate delivery of target mRNA to distal neuronal compartments. TDP-43 mutations impair this mRNA transport function in vivo and in vitro, including in stem cell-derived motor neurons from ALS patients bearing any one of three different TDP-43 ALS-causing mutations. Thus, TDP-43 mutations that cause ALS lead to partial loss of a novel cytoplasmic function of TDP-43.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Al-Chalabi A, Jones A, Troakes C, King A, Al-Sarraj S, van den Berg LH. The genetics and neuropathology of amyotrophic lateral sclerosis. Acta neuropathologica. 2012;124:339–352. - PubMed
-
- Arnold ES, Ling SC, Huelga SC, Lagier-Tourenne C, Polymenidou M, Ditsworth D, Kordasiewicz HB, McAlonis-Downes M, Platoshyn O, Parone PA, et al. ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43. Proc Natl Acad Sci U S A. 2013;110:E736–745. - PMC - PubMed
-
- Bilican B, Serio A, Barmada SJ, Nishimura AL, Sullivan GJ, Carrasco M, Phatnani HP, Puddifoot CA, Story D, Fletcher J, et al. Mutant induced pluripotent stem cell lines recapitulate aspects of TDP-43 proteinopathies and reveal cell-specific vulnerability. Proc Natl Acad Sci U S A. 2012;109:5803–5808. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS053825/NS/NINDS NIH HHS/United States
- G0300329/MRC_/Medical Research Council/United Kingdom
- 089701/WT_/Wellcome Trust/United Kingdom
- G0900635/MRC_/Medical Research Council/United Kingdom
- DP1 NS082099/NS/NINDS NIH HHS/United States
- MC_G1000733/MRC_/Medical Research Council/United Kingdom
- P30 CA021765-34/CA/NCI NIH HHS/United States
- G0600974/MRC_/Medical Research Council/United Kingdom
- AG031587/AG/NIA NIH HHS/United States
- G1100695/MRC_/Medical Research Council/United Kingdom
- R01 AG031587/AG/NIA NIH HHS/United States
- 8DP1NS082099/DP/NCCDPHP CDC HHS/United States
- CHANDRAN/MAR10/982-797/MNDA_/Motor Neurone Disease Association/United Kingdom
- G0501573/MRC_/Medical Research Council/United Kingdom
- R01 NS053825/NS/NINDS NIH HHS/United States
- G0500289/MRC_/Medical Research Council/United Kingdom
- P30 CA021765/CA/NCI NIH HHS/United States
- G0900688/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
