Calcium signaling in lacrimal glands
- PMID: 24507443
- PMCID: PMC4058340
- DOI: 10.1016/j.ceca.2014.01.001
Calcium signaling in lacrimal glands
Abstract
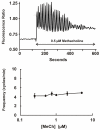
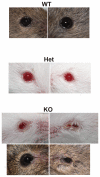
Lacrimal glands provide the important function of lubricating and protecting the ocular surface. Failure of proper lacrimal gland function results in a number of debilitating dry eye diseases. Lacrimal glands secrete lipids, mucins, proteins, salts and water and these secretions are at least partially regulated by neurotransmitter-mediated cell signaling. The predominant signaling mechanism for lacrimal secretion involves activation of phospholipase C, generation of the Ca(2+)-mobilizing messenger, IP3, and release of Ca(2+) stored in the endoplasmic reticulum. The loss of Ca(2+) from the endoplasmic reticulum then triggers a process known as store-operated Ca(2+) entry, involving a Ca(2+) sensor in the endoplasmic reticulum, STIM1, which activates plasma membrane store-operated channels comprised of Orai subunits. Recent studies with deletions of the channel subunit, Orai1, confirm the important role of SOCE in both fluid and protein secretion in lacrimal glands, both in vivo and in vitro.
Keywords: Calcium entry; Calcium oscillations; Calcium release; Calcium signaling; Fluid secretion; IP3; Lacrimal; Orai; Protein secretion; STIM.
Published by Elsevier Ltd.
Figures


References
-
- Dartt DA. Dysfunctional neural regulation of lacrimal gland secretion and its role in the pathogenesis of dry eye syndromes. Ocul Surf. 2004;2:76–91. - PubMed
-
- Bothelo SY. Tears and the lacrimal gland. Scientific American. 1964;211:78–86. - PubMed
-
- Bothelo SY, Hisada M, Fuenmayor N. Functional innervation of the lacrimal gland in the cat. Origin of secretomotor fibers in the lacrimal nerve. Arch Opthalmol. 1966;76:581–588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

