The wall-stress footprint of blood cells flowing in microvessels
- PMID: 24507616
- PMCID: PMC3944993
- DOI: 10.1016/j.bpj.2013.12.020
The wall-stress footprint of blood cells flowing in microvessels
Abstract
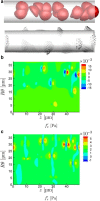
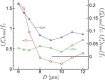
It is well known that mechanotransduction of hemodynamic forces mediates cellular processes, particularly those that lead to vascular development and maintenance. Both the strength and space-time character of these forces have been shown to affect remodeling and morphogenesis. However, the role of blood cells in the process remains unclear. We investigate the possibility that in the smallest vessels blood's cellular character of itself will lead to forces fundamentally different than the time-averaged forces usually considered, with fluctuations that may significantly exceed their mean values. This is quantitated through the use of a detailed simulation model of microvessel flow in two principal configurations: a diameter D=6.5 μm tube-a model for small capillaries through which red blood cells flow in single-file-and a D=12 μm tube-a model for a nascent vein or artery through which the cells flow in a confined yet chaotic fashion. Results in both cases show strong sensitivity to the mean flow speed U. Peak stresses exceed their means by greater than a factor of 10 when U/D≲10 s(-1), which corresponds to the inverse relaxation time of a healthy red blood cell. This effect is more significant for smaller D cases. At faster flow rates, including those more commonly observed under normal, nominally static physiological conditions, the peak fluctuations are more comparable with the mean shear stress. Implications for mechanotransduction of hemodynamic forces are discussed.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures









References
-
- Chapman W.B. The effect of the heart-beat upon the development of the vascular system in the chick. Am. J. Anat. 1918;23:175–203.
-
- Wang L., Zhang P., Liu F. A blood flow-dependent klf2a-NO signaling cascade is required for stabilization of hematopoietic stem cell programming in zebrafish embryos. Blood. 2011;118:4102–4110. - PubMed
-
- Liu D., Krueger J., Le Noble F. The role of blood flow and microRNAs in blood vessel development. Int. J. Dev. Biol. 2011;55:419–429. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

