Solving glycosylation disorders: fundamental approaches reveal complicated pathways
- PMID: 24507773
- PMCID: PMC3928651
- DOI: 10.1016/j.ajhg.2013.10.024
Solving glycosylation disorders: fundamental approaches reveal complicated pathways
Abstract
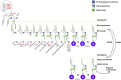
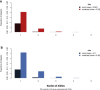
Over 100 human genetic disorders result from mutations in glycosylation-related genes. In 2013, a new glycosylation disorder was reported every 17 days. This trend will probably continue given that at least 2% of the human genome encodes glycan-biosynthesis and -recognition proteins. Established biosynthetic pathways provide many candidate genes, but finding unanticipated mutated genes will offer new insights into glycosylation. Simple glycobiomarkers can be used in narrowing the candidates identified by exome and genome sequencing, and those can be validated by glycosylation analysis of serum or cells from affected individuals. Model organisms will expand the understanding of these mutations' impact on glycosylation and pathology. Here, we highlight some recently discovered glycosylation disorders and the barriers, breakthroughs, and surprises they presented. We predict that some glycosylation disorders might occur with greater frequency than current estimates of their prevalence. Moreover, the prevalence of some disorders differs substantially between European and African Americans.
Copyright © 2014 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- de Ligt J., Willemsen M.H., van Bon B.W., Kleefstra T., Yntema H.G., Kroes T., Vulto-van Silfhout A.T., Koolen D.A., de Vries P., Gilissen C. Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 2012;367:1921–1929. - PubMed
-
- Mefford H.C. Diagnostic exome sequencing—are we there yet? N. Engl. J. Med. 2012;367:1951–1953. - PubMed
-
- Varki A., Lowe J.B. Biological Roles of Glycans. In: Varki A., Cummings R.D., Esko J.D., Freeze H.H., Stanley P., Bertozzi C.R., Hart G.W., Etzler M.E., editors. Essentials of Glycobiology. Second Edition. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2009. pp. 75–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

