Emerging roles of hematopoietic cells in the pathobiology of diabetic complications
- PMID: 24507996
- PMCID: PMC3975817
- DOI: 10.1016/j.tem.2014.01.002
Emerging roles of hematopoietic cells in the pathobiology of diabetic complications
Abstract
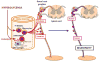
Diabetic complications encompass macrovascular events, mainly the result of accelerated atherosclerosis, and microvascular events that strike the eye (retinopathy), kidney (nephropathy), and nervous system (neuropathy). The traditional view is that hyperglycemia-induced dysregulated biochemical pathways cause injury and death of cells intrinsic to the organs affected. There is emerging evidence that diabetes compromises the function of the bone marrow (BM), producing a stem cell niche-dependent defect in hematopoietic stem cell mobilization. Furthermore, dysfunctional BM-derived hematopoietic cells contribute to diabetic complications. Thus, BM cells are not only a victim but also an accomplice in diabetes and diabetic complications. Understanding the underlying molecular mechanisms may lead to the development of new therapies to prevent and/or treat diabetic complications by specifically targeting these perpetrators.
Keywords: bone marrow; complications; diabetes mellitus; diabetic nephropathy; diabetic neuropathy; diabetic retinopathy; hematopoietic cells.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


References
-
- Brownlee M. The pathobiology of diabetic complications a unifying mechanism. Diabetes. 2005;54:1615–1625. - PubMed
-
- Manigrasso MB, et al. Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol Metab. 2013 http://dx.doi.org/10.1016/j.tem.2013.08.002. - DOI - PMC - PubMed
-
- Fadini GP, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. - PubMed
-
- Fadini GP, et al. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia. 2006;49:3075–3084. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

