Honokiol inhibits epithelial-mesenchymal transition in breast cancer cells by targeting signal transducer and activator of transcription 3/Zeb1/E-cadherin axis
- PMID: 24508063
- PMCID: PMC4009450
- DOI: 10.1016/j.molonc.2014.01.004
Honokiol inhibits epithelial-mesenchymal transition in breast cancer cells by targeting signal transducer and activator of transcription 3/Zeb1/E-cadherin axis
Abstract
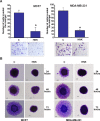
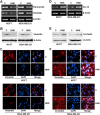
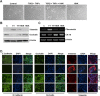
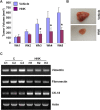
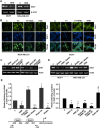
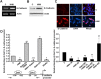
Epithelial-mesenchymal transition (EMT), a critical step in the acquisition of metastatic state, is an attractive target for therapeutic interventions directed against tumor metastasis. Honokiol (HNK) is a natural phenolic compound isolated from an extract of seed cones from Magnolia grandiflora. Recent studies from our lab show that HNK impedes breast carcinogenesis. Here, we provide molecular evidence that HNK inhibits EMT in breast cancer cells resulting in significant downregulation of mesenchymal marker proteins and concurrent upregulation of epithelial markers. Experimental EMT induced by exposure to TGFβ and TNFα in spontaneously immortalized nontumorigenic human mammary epithelial cells is also completely reversed by HNK as evidenced by morphological as well as molecular changes. Investigating the downstream mediator(s) that may direct EMT inhibition by HNK, we found functional interactions between HNK, Stat3, and EMT-signaling components. In vitro and in vivo analyses show that HNK inhibits Stat3 activation in breast cancer cells and tumors. Constitutive activation of Stat3 abrogates HNK-mediated activation of epithelial markers whereas inhibition of Stat3 using small molecule inhibitor, Stattic, potentiates HNK-mediated inhibition of EMT markers, invasion and migration of breast cancer cells. Mechanistically, HNK inhibits recruitment of Stat3 on mesenchymal transcription factor Zeb1 promoter resulting in decreased Zeb1 expression and nuclear translocation. We also discover that HNK increases E-cadherin expression via Stat3-mediated release of Zeb1 from E-cadherin promoter. Collectively, this study reports that HNK effectively inhibits EMT in breast cancer cells and provide evidence for a previously unrecognized cross-talk between HNK and Stat3/Zeb1/E-cadherin axis.
Keywords: Breast cancer; E-cadherin; EMT; Honokiol; Stat3; Zeb1.
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Ahn, K.S. , Sethi, G. , Shishodia, S. , Sung, B. , Arbiser, J.L. , Aggarwal, B.B. , 2006. Honokiol potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through modulation of nuclear factor-kappaB activation pathway. Mol. Cancer Res. MCR. 4, 621–633. - PubMed
-
- Bai, X. , Cerimele, F. , Ushio-Fukai, M. , Waqas, M. , Campbell, P.M. , Govindarajan, B. , Der, C.J. , Battle, T. , Frank, D.A. , Ye, K. , Murad, E. , Dubiel, W. , Soff, G. , Arbiser, J.L. , 2003. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J. Biol. Chem.. 278, 35501–35507. - PubMed
-
- Bar-Sela, G. , Epelbaum, R. , Schaffer, M. , 2010. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr. Med. Chem.. 17, 190–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

