Thromboprophylaxis with fondaparinux in high-risk postoperative patients with renal insufficiency
- PMID: 24508189
- PMCID: PMC4156854
- DOI: 10.1016/j.thromres.2013.11.019
Thromboprophylaxis with fondaparinux in high-risk postoperative patients with renal insufficiency
Abstract
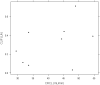
Fondaparinux is an antithrombin-dependent factor Xa inhibitor that is used for thromboprophylaxis of patients undergoing hip fracture surgery, hip or knee replacement, or abdominal surgery. It is cleared by the kidney and should be used with caution in patients with renal impairment and avoided in patients with severe renal insufficiency. Recently, several studies have demonstrated that a lower dose of fondaparinux in patients with moderate renal impairment appears to be safe and effective. The purpose of this study was to obtain pharmacokinetic and clinical data on the use of prophylactic fondaparinux in patients with renal insufficiency undergoing major abdominal surgery for cancer (n=8) or orthopedic surgery (n=1). Anti-factor Xa levels were obtained, and a published population pharmacokinetic model for fondaparinux was fit to the data. The data were analyzed using NONMEM software. Fondaparinux did not appear to accumulate in these patients, even when the drug was administered for up to twelve days. Pharmacokinetic analysis revealed that the apparent clearance in this population, who were primarily undergoing cancer surgery, was similar to prior studies in orthopedic surgery patients. In contrast, lower estimates were obtained for volume of distribution and absorption rate constant parameters. None of the patients sustained a hemorrhagic complication attributable to fondaparinux. One patient developed hypoxia in the setting of transient atrial fibrillation and clinical suspicion for pulmonary embolism, but this was not confirmed radiographically. These results support the use of 1.5mg of fondaparinux every 24hours for thromboprophylaxis in patients with renal insufficiency undergoing high-risk surgical procedures.
Keywords: Fondaparinux; Renal failure; Thromboprophylaxis.
Copyright © 2013. Published by Elsevier Ltd.
Conflict of interest statement
Figures

References
-
- Agnelli G, Bergqvist D, Cohen AT, Gallus AS, Gent M. Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high-risk abdominal surgery. Br J Surg. 2005;92(10):1212–20. - PubMed
-
- Turpie AG, Bauer KA, Eriksson BI, Lassen MR. Postoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip-replacement surgery: a randomised double-blind trial. Lancet. 2002;359(9319):1721–6. - PubMed
-
- Eriksson BI, Bauer KA, Lassen MR, Turpie AG. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N Engl J Med. 2001;345(18):1298–304. - PubMed
-
- Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell CW., Jr . Chest. 9th. 2 Suppl. Vol. 141. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines; 2012. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis; pp. e278S–325S. - PMC - PubMed
-
- Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, Samama CM. Chest. 9th. 2 Suppl. Vol. 141. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines; 2012. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis; pp. e227S–77S. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

