F-box and leucine-rich repeat protein 5 (FBXL5): sensing intracellular iron and oxygen
- PMID: 24508277
- PMCID: PMC3959624
- DOI: 10.1016/j.jinorgbio.2014.01.015
F-box and leucine-rich repeat protein 5 (FBXL5): sensing intracellular iron and oxygen
Abstract
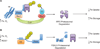
Though essential for many vital biological processes, excess iron results in the formation of damaging reactive oxygen species (ROS). Therefore, iron metabolism must be tightly regulated. F-box and leucine-rich repeat protein 5 (FBXL5), an E3 ubiquitin ligase subunit, regulates cellular and systemic iron homeostasis by facilitating iron regulatory protein 2 (IRP2) degradation. FBXL5 possesses an N-terminal hemerythrin (Hr)-like domain that mediates its own differential stability by switching between two different conformations to communicate cellular iron availability. In addition, the FBXL5-Hr domain also senses O2 availability, albeit by a distinct mechanism. Mice lacking FBXL5 fail to sense intracellular iron levels and die in utero due to iron overload and exposure to damaging levels of oxidative stress. By closely monitoring intracellular levels of iron and oxygen, FBLX5 prevents the formation of conditions that favor ROS formation. These findings suggest that FBXL5 is essential for the maintenance of iron homeostasis and is a key sensor of bioavailable iron. Here, we describe the iron and oxygen sensing mechanisms of the FBXL5 Hr-like domain and its role in mediating ROS biology.
Keywords: FBXL5; Hemerythrin; Iron; Iron regulatory proteins; Oxygen; Reactive oxygen species.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



References
-
- Crichton RR, Pierre JL. Biometals. 2001;14:99–112. - PubMed
-
- Klausner RD, Rouault TA, Harford JB. Cell. 1993;72:19–28. - PubMed
-
- Cotton FA, Wilkinson G, Murillo CA, Bochmann M. Advanced Inorganic Chemistry. sixth ed. New Jersey: Wiley-Interscience; 1999.
-
- Brzoska K, Meczynska S, Kruszewski M. Acta. Biochim. Pol. 2006;53:685–691. - PubMed
-
- Perutz MF. Harvey Lect. 1969;63:213–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

