Cell-nonautonomous effects of dFOXO/DAF-16 in aging
- PMID: 24508462
- PMCID: PMC3969275
- DOI: 10.1016/j.celrep.2014.01.015
Cell-nonautonomous effects of dFOXO/DAF-16 in aging
Abstract
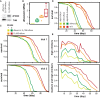
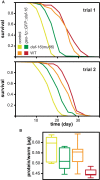
Drosophila melanogaster and Caenorhabditis elegans each carry a single representative of the Forkhead box O (FoxO) family of transcription factors, dFOXO and DAF-16, respectively. Both are required for lifespan extension by reduced insulin/Igf signaling, and their activation in key tissues can extend lifespan. Aging of these tissues may limit lifespan. Alternatively, FoxOs may promote longevity cell nonautonomously by signaling to themselves (FoxO to FoxO) or other factors (FoxO to other) in distal tissues. Here, we show that activation of dFOXO and DAF-16 in the gut/fat body does not require dfoxo/daf-16 elsewhere to extend lifespan. Rather, in Drosophila, activation of dFOXO in the gut/fat body or in neuroendocrine cells acts on other organs to promote healthy aging by signaling to other, as-yet-unidentified factors. Whereas FoxO-to-FoxO signaling appears to be required for metabolic homeostasis, our results pinpoint FoxO-to-other signaling as an important mechanism through which localized FoxO activity ameliorates aging.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

