SN79, a sigma receptor antagonist, attenuates methamphetamine-induced astrogliosis through a blockade of OSMR/gp130 signaling and STAT3 phosphorylation
- PMID: 24508558
- PMCID: PMC4241368
- DOI: 10.1016/j.expneurol.2014.01.020
SN79, a sigma receptor antagonist, attenuates methamphetamine-induced astrogliosis through a blockade of OSMR/gp130 signaling and STAT3 phosphorylation
Abstract
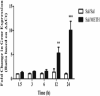
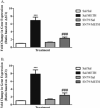
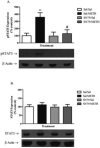
Methamphetamine (METH) exposure results in dopaminergic neurotoxicity in striatal regions of the brain, an effect that has been linked to an increased risk of Parkinson's disease. Various aspects of neuroinflammation, including astrogliosis, are believed to be contributory factors in METH neurotoxicity. METH interacts with sigma receptors at physiologically relevant concentrations and treatment with sigma receptor antagonists has been shown to mitigate METH-induced neurotoxicity in rodent models. Whether these compounds alter the responses of glial cells within the central nervous system to METH however has yet to be determined. Therefore, the purpose of the current study was to determine whether the sigma receptor antagonist, SN79, mitigates METH-induced striatal reactive astrogliosis. Male, Swiss Webster mice treated with a neurotoxic regimen of METH exhibited time-dependent increases in striatal gfap mRNA and concomitant increases in GFAP protein, indicative of astrogliosis. This is the first report that similar to other neurotoxicants that induce astrogliosis through the activation of JAK2/STAT3 signaling by stimulating gp-130-linked cytokine signaling resulting from neuroinflammation, METH treatment also increases astrocytic oncostatin m receptor (OSMR) expression and the phosphorylation of STAT3 (Tyr-705) in vivo. Pretreatment with SN79 blocked METH-induced increases in OSMR, STAT3 phosphorylation and astrocyte activation within the striatum. Additionally, METH treatment resulted in striatal cellular degeneration as measured by Fluoro-Jade B, an effect that was mitigated by SN79. The current study provides evidence that sigma receptor antagonists attenuate METH-induced astrocyte activation through a pathway believed to be shared by various neurotoxicants.
Keywords: Astrocyte; Glia; Methamphetamine neurotoxicity; Neuroinflammation; Sigma receptor.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
The Crosstalk Between Neurons and Glia in Methamphetamine-Induced Neuroinflammation.Neurochem Res. 2022 Apr;47(4):872-884. doi: 10.1007/s11064-021-03513-9. Epub 2022 Jan 4. Neurochem Res. 2022. PMID: 34982394 Review.
-
SN79, a sigma receptor ligand, blocks methamphetamine-induced microglial activation and cytokine upregulation.Exp Neurol. 2013 Sep;247:134-42. doi: 10.1016/j.expneurol.2013.04.009. Epub 2013 Apr 28. Exp Neurol. 2013. PMID: 23631864 Free PMC article.
-
Pharmacological evaluation of SN79, a sigma (σ) receptor ligand, against methamphetamine-induced neurotoxicity in vivo.Eur Neuropsychopharmacol. 2013 Aug;23(8):960-71. doi: 10.1016/j.euroneuro.2012.08.005. Epub 2012 Aug 24. Eur Neuropsychopharmacol. 2013. PMID: 22921523 Free PMC article.
-
σ Receptor antagonist attenuation of methamphetamine-induced neurotoxicity is correlated to body temperature modulation.Pharmacol Rep. 2013;65(2):343-9. doi: 10.1016/s1734-1140(13)71009-0. Pharmacol Rep. 2013. PMID: 23744418
-
The Role of α-Synuclein in Methamphetamine-Induced Neurotoxicity.Neurotox Res. 2021 Jun;39(3):1007-1021. doi: 10.1007/s12640-021-00332-2. Epub 2021 Feb 8. Neurotox Res. 2021. PMID: 33555547 Review.
Cited by
-
The regulatory role of endoplasmic reticulum chaperone proteins in neurodevelopment.Front Neurosci. 2022 Nov 15;16:1032607. doi: 10.3389/fnins.2022.1032607. eCollection 2022. Front Neurosci. 2022. PMID: 36458041 Free PMC article. Review.
-
Oncostatin M promotes excitotoxicity by inhibiting glutamate uptake in astrocytes: implications in HIV-associated neurotoxicity.J Neuroinflammation. 2016 Jun 10;13(1):144. doi: 10.1186/s12974-016-0613-8. J Neuroinflammation. 2016. PMID: 27287400 Free PMC article.
-
Involvement of sigma-1 receptor in astrocyte activation induced by methamphetamine via up-regulation of its own expression.J Neuroinflammation. 2015 Feb 17;12:29. doi: 10.1186/s12974-015-0250-7. J Neuroinflammation. 2015. PMID: 25889537 Free PMC article.
-
Methamphetamine Augments Concurrent Astrocyte Mitochondrial Stress, Oxidative Burden, and Antioxidant Capacity: Tipping the Balance in HIV-Associated Neurodegeneration.Neurotox Res. 2018 Feb;33(2):433-447. doi: 10.1007/s12640-017-9812-z. Epub 2017 Oct 9. Neurotox Res. 2018. PMID: 28993979 Free PMC article.
-
The Crosstalk Between Neurons and Glia in Methamphetamine-Induced Neuroinflammation.Neurochem Res. 2022 Apr;47(4):872-884. doi: 10.1007/s11064-021-03513-9. Epub 2022 Jan 4. Neurochem Res. 2022. PMID: 34982394 Review.
References
-
- Ajmo CT, Jr., Vernon DO, Collier L, Pennypacker KR, Cuevas J. Sigma receptor activation reduces infarct size at 24 hours after permanent middle cerebral artery occlusion in rats. Curr Neurovasc Res. 2006;3:89–98. - PubMed
-
- Anderson KJ, Fugaccia I, Scheff SW. Fluoro-jade B stains quiescent and reactive astrocytes in the rodent spinal cord. J Neurotrauma. 2003;20:1223–31. - PubMed
-
- Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–32. - PubMed
-
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr., Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

