Role for myosin-V motor proteins in the selective delivery of Kv channel isoforms to the membrane surface of cardiac myocytes
- PMID: 24508725
- PMCID: PMC4213814
- DOI: 10.1161/CIRCRESAHA.114.302711
Role for myosin-V motor proteins in the selective delivery of Kv channel isoforms to the membrane surface of cardiac myocytes
Abstract
Rationale: Kv1.5 (KCNA5) mediates the ultra-rapid delayed rectifier current that controls atrial action potential duration. Given its atrial-specific expression and alterations in human atrial fibrillation, Kv1.5 has emerged as a promising target for the treatment of atrial fibrillation. A necessary step in the development of novel agents that selectively modulate trafficking pathways is the identification of the cellular machinery controlling Kv1.5 surface density, of which little is yet known.
Objective: To investigate the role of the unconventional myosin-V (MYO5A and MYO5B) motors in determining the cell surface density of Kv1.5.
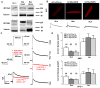
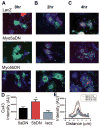
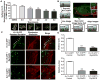
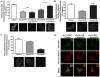
Methods and results: Western blot analysis showed MYO5A and MYO5B expression in the heart, whereas disruption of endogenous motors selectively reduced IKur current in adult rat cardiomyocytes. Dominant negative constructs and short hairpin RNA silencing demonstrated a role for MYO5A and MYO5B in the surface trafficking of Kv1.5 and connexin-43 but not potassium voltage-gated channel, subfamily H (eag-related), member 2 (KCNH2). Live-cell imaging of Kv1.5-GFP and retrospective labeling of phalloidin demonstrated motility of Kv1.5 vesicles on actin tracts. MYO5A participated in anterograde trafficking, whereas MYO5B regulated postendocytic recycling. Overexpression of mutant motors revealed a selective role for Rab11 in coupling MYO5B to Kv1.5 recycling.
Conclusions: MYO5A and MYO5B control functionally distinct steps in the surface trafficking of Kv1.5. These isoform-specific trafficking pathways determine Kv1.5-encoded IKur in myocytes to regulate repolarizing current and, consequently, cardiac excitability. Therapeutic strategies that manipulate Kv1.5 selective trafficking pathways may prove useful in the treatment of arrhythmias.
Keywords: Kv1.5 potassium channel; arrhythmias, cardiac; connexin 43; heart.
Figures




Similar articles
-
Microtubule polymerization state and clathrin-dependent internalization regulate dynamics of cardiac potassium channel: Microtubule and clathrin control of KV1.5 channel.J Mol Cell Cardiol. 2020 Jul;144:127-139. doi: 10.1016/j.yjmcc.2020.05.004. Epub 2020 May 20. J Mol Cell Cardiol. 2020. PMID: 32445844
-
The anchoring protein SAP97 retains Kv1.5 channels in the plasma membrane of cardiac myocytes.Am J Physiol Heart Circ Physiol. 2008 Apr;294(4):H1851-61. doi: 10.1152/ajpheart.01045.2007. Epub 2008 Feb 1. Am J Physiol Heart Circ Physiol. 2008. PMID: 18245566
-
Cardiac dysfunction related to cardiac mRNA and protein traffic impairment due to reduced unconventional motor protein myosin-5b expression.Eur Heart J. 2025 Jul 1;46(25):2437-2454. doi: 10.1093/eurheartj/ehaf047. Eur Heart J. 2025. PMID: 39969162 Free PMC article.
-
Challenges Faced with Small Molecular Modulators of Potassium Current Channel Isoform Kv1.5.Biomolecules. 2019 Dec 19;10(1):10. doi: 10.3390/biom10010010. Biomolecules. 2019. PMID: 31861703 Free PMC article. Review.
-
Pharmacology of voltage-gated potassium channel Kv1.5--impact on cardiac excitability.Curr Opin Pharmacol. 2014 Apr;15:115-21. doi: 10.1016/j.coph.2014.02.001. Epub 2014 Mar 13. Curr Opin Pharmacol. 2014. PMID: 24632326 Review.
Cited by
-
SnRNA sequencing defines signaling by RBC-derived extracellular vesicles in the murine heart.Life Sci Alliance. 2021 Oct 18;4(12):e202101048. doi: 10.26508/lsa.202101048. Print 2021 Dec. Life Sci Alliance. 2021. PMID: 34663679 Free PMC article.
-
The Pattern of mRNA Expression Is Changed in Sinoatrial Node from Goto-Kakizaki Type 2 Diabetic Rat Heart.J Diabetes Res. 2018 Sep 2;2018:8454078. doi: 10.1155/2018/8454078. eCollection 2018. J Diabetes Res. 2018. PMID: 30246030 Free PMC article.
-
SAP97 and cortactin remodeling in arrhythmogenic Purkinje cells.PLoS One. 2014 Sep 3;9(9):e106830. doi: 10.1371/journal.pone.0106830. eCollection 2014. PLoS One. 2014. PMID: 25184222 Free PMC article.
-
Inhibitory effects of cholinesterase inhibitor donepezil on the Kv1.5 potassium channel.Sci Rep. 2017 Feb 13;7:41509. doi: 10.1038/srep41509. Sci Rep. 2017. PMID: 28198801 Free PMC article.
-
Ion channel traffic jams: the significance of trafficking deficiency in long QT syndrome.Cell Discov. 2025 Jan 10;11(1):3. doi: 10.1038/s41421-024-00738-0. Cell Discov. 2025. PMID: 39788950 Free PMC article. Review.
References
-
- Camm AJ. Safety considerations in the pharmacological management of atrial fibrillation. Int J Cardiol. 2008;127:299–306. - PubMed
-
- Camm AJ, Savelieva I. Advances in antiarrhythmic drug treatment of atrial fibrillation: Where do we stand now? Heart Rhythm. 2004;1:244–246. - PubMed
-
- Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, Bergmann JF. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane database of systematic reviews (Online) 2007:CD005049. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

