Activated alveolar epithelial cells initiate fibrosis through autocrine and paracrine secretion of connective tissue growth factor
- PMID: 24508728
- PMCID: PMC3989723
- DOI: 10.1152/ajplung.00243.2013
Activated alveolar epithelial cells initiate fibrosis through autocrine and paracrine secretion of connective tissue growth factor
Abstract
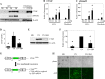
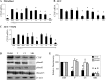
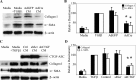
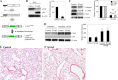
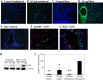
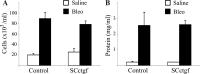
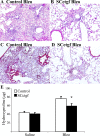
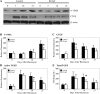
Fibrogenesis involves a pathological accumulation of activated fibroblasts and extensive matrix remodeling. Profibrotic cytokines, such as TGF-β, stimulate fibroblasts to overexpress fibrotic matrix proteins and induce further expression of profibrotic cytokines, resulting in progressive fibrosis. Connective tissue growth factor (CTGF) is a profibrotic cytokine that is indicative of fibroblast activation. Epithelial cells are abundant in the normal lung, but their contribution to fibrogenesis remains poorly defined. Profibrotic cytokines may activate epithelial cells with protein expression and functions that overlap with the functions of active fibroblasts. We found that alveolar epithelial cells undergoing TGF-β-mediated mesenchymal transition in vitro were also capable of activating lung fibroblasts through production of CTGF. Alveolar epithelial cell expression of CTGF was dramatically reduced by inhibition of Rho signaling. CTGF reporter mice demonstrated increased CTGF promoter activity by lung epithelial cells acutely after bleomycin in vivo. Furthermore, mice with lung epithelial cell-specific deletion of CTGF had an attenuated fibrotic response to bleomycin. These studies provide direct evidence that epithelial cell activation initiates a cycle of fibrogenic effector cell activation during progressive fibrosis. Therapy targeted at epithelial cell production of CTGF offers a novel pathway for abrogating this progressive cycle and limiting tissue fibrosis.
Keywords: connective tissue growth factor; epithelial; epithelial-mesenchymal transition; fibrosis; lung.
Figures
Similar articles
-
Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF.Eur Respir J. 2001 Jun;17(6):1220-7. doi: 10.1183/09031936.01.00074101. Eur Respir J. 2001. PMID: 11491168
-
Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of type I collagen.Arthritis Rheum. 2009 Jul;60(7):2142-55. doi: 10.1002/art.24620. Arthritis Rheum. 2009. PMID: 19565505
-
TGFβ signaling in lung epithelium regulates bleomycin-induced alveolar injury and fibroblast recruitment.Am J Physiol Lung Cell Mol Physiol. 2011 Jun;300(6):L887-97. doi: 10.1152/ajplung.00397.2010. Epub 2011 Mar 25. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21441353 Free PMC article.
-
Sphingolipids in pulmonary fibrosis.Adv Biol Regul. 2015 Jan;57:55-63. doi: 10.1016/j.jbior.2014.09.008. Epub 2014 Oct 13. Adv Biol Regul. 2015. PMID: 25446881 Free PMC article. Review.
-
Drugs targeting CTGF in the treatment of pulmonary fibrosis.J Cell Mol Med. 2024 May;28(10):e18448. doi: 10.1111/jcmm.18448. J Cell Mol Med. 2024. PMID: 38774993 Free PMC article. Review.
Cited by
-
Comprehensive Bioinformatics Identifies Key microRNA Players in ATG7-Deficient Lung Fibroblasts.Int J Mol Sci. 2020 Jun 9;21(11):4126. doi: 10.3390/ijms21114126. Int J Mol Sci. 2020. PMID: 32527064 Free PMC article.
-
TGF-β1-induced deposition of provisional extracellular matrix by tracheal basal cells promotes epithelial-to-mesenchymal transition in a c-Jun NH2-terminal kinase-1-dependent manner.Am J Physiol Lung Cell Mol Physiol. 2018 Jun 1;314(6):L984-L997. doi: 10.1152/ajplung.00053.2017. Epub 2018 Feb 22. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29469614 Free PMC article.
-
TRIB3 promotes pulmonary fibrosis through inhibiting SLUG degradation by physically interacting with MDM2.Acta Pharm Sin B. 2023 Apr;13(4):1631-1647. doi: 10.1016/j.apsb.2023.01.008. Epub 2023 Jan 11. Acta Pharm Sin B. 2023. PMID: 37139431 Free PMC article.
-
Increased Epithelial Expression of CTGF and S100A7 with Elevated Subepithelial Expression of IL-1β in Trachomatous Trichiasis.PLoS Negl Trop Dis. 2016 Jun 1;10(6):e0004752. doi: 10.1371/journal.pntd.0004752. eCollection 2016 Jun. PLoS Negl Trop Dis. 2016. PMID: 27249027 Free PMC article.
-
CD36/Lyn kinase interactions within macrophages promotes pulmonary fibrosis in response to oxidized phospholipid.Respir Res. 2023 Dec 14;24(1):314. doi: 10.1186/s12931-023-02629-6. Respir Res. 2023. PMID: 38098035 Free PMC article.
References
-
- American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment International consensus statement American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 161: 646–664, 2000 - PubMed
-
- American Thoracic Society/European Respiratory Society. International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 165: 277–304, 2002 - PubMed
-
- Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest 125: 754–765, 2004 - PubMed
-
- Bauman KA, Wettlaufer SH, Okunishi K, Vannella KM, Stoolman JS, Huang SK, Courey AJ, White ES, Hogaboam CM, Simon RH, Toews GB, Sisson TH, Moore BB, Peters-Golden M. The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice. J Clin Invest 120: 1950–1960, 2010 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

