High proliferative potential endothelial colony-forming cells contribute to hypoxia-induced pulmonary artery vasa vasorum neovascularization
- PMID: 24508729
- PMCID: PMC3962625
- DOI: 10.1152/ajplung.00244.2013
High proliferative potential endothelial colony-forming cells contribute to hypoxia-induced pulmonary artery vasa vasorum neovascularization
Abstract
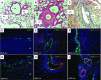
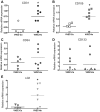
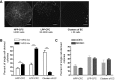
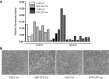
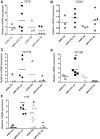
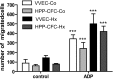
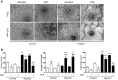
Angiogenic expansion of the vasa vasorum (VV) is an important contributor to pulmonary vascular remodeling in the pathogenesis of pulmonary hypertension (PH). High proliferative potential endothelial progenitor-like cells have been described in vascular remodeling and angiogenesis in both systemic and pulmonary circulations. However, their role in hypoxia-induced pulmonary artery (PA) VV expansion in PH is not known. We hypothesized that profound PA VV neovascularization observed in a neonatal calf model of hypoxia-induced PH is due to increased numbers of subsets of high proliferative cells within the PA adventitial VV endothelial cells (VVEC). Using a single cell clonogenic assay, we found that high proliferative potential colony-forming cells (HPP-CFC) comprise a markedly higher percentage in VVEC populations isolated from the PA of hypoxic (VVEC-Hx) compared with control (VVEC-Co) calves. VVEC-Hx populations that comprised higher numbers of HPP-CFC also demonstrated markedly higher expression levels of CD31, CD105, and c-kit than VVEC-Co. In addition, significantly higher expression of CD31, CD105, and c-kit was observed in HPP-CFC vs. the VVEC of the control but not of hypoxic animals. HPP-CFC exhibited migratory and tube formation capabilities, two important attributes of angiogenic phenotype. Furthermore, HPP-CFC-Co and some HPP-CFC-Hx exhibited elevated telomerase activity, consistent with their high replicative potential, whereas a number of HPP-CFC-Hx exhibited impaired telomerase activity, suggestive of their senescence state. In conclusion, our data suggest that hypoxia-induced VV expansion involves an emergence of HPP-CFC populations of a distinct phenotype with increased angiogenic capabilities. These cells may serve as a potential target for regulating VVEC neovascularization.
Keywords: endothelial clusters; endothelial progenitor cells; high proliferative potential endothelial colony-forming cells; pulmonary hypertension; vascular remodeling.
Figures
Similar articles
-
Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells.Am J Physiol Lung Cell Mol Physiol. 2004 Apr;286(4):L668-78. doi: 10.1152/ajplung.00108.2003. Epub 2003 May 16. Am J Physiol Lung Cell Mol Physiol. 2004. PMID: 12754186
-
Isolation of vasa vasorum endothelial cells from pulmonary artery adventitia: Implementation to vascular biology research.Microvasc Res. 2023 May;147:104479. doi: 10.1016/j.mvr.2023.104479. Epub 2023 Jan 21. Microvasc Res. 2023. PMID: 36690271
-
c-Jun, Foxo3a, and c-Myc Transcription Factors are Key Regulators of ATP-Mediated Angiogenic Responses in Pulmonary Artery Vasa Vasorum Endothelial Cells.Cells. 2020 Feb 11;9(2):416. doi: 10.3390/cells9020416. Cells. 2020. PMID: 32054096 Free PMC article.
-
Vasa vasorum and atherosclerosis - Quid novi?Thromb Haemost. 2007 Jun;97(6):873-9. Thromb Haemost. 2007. PMID: 17549287 Review.
-
Vasa vasorum in atherosclerosis and clinical significance.Int J Mol Sci. 2015 May 20;16(5):11574-608. doi: 10.3390/ijms160511574. Int J Mol Sci. 2015. PMID: 26006236 Free PMC article. Review.
Cited by
-
On vasa vasorum: A history of advances in understanding the vessels of vessels.Sci Adv. 2022 Apr 22;8(16):eabl6364. doi: 10.1126/sciadv.abl6364. Epub 2022 Apr 20. Sci Adv. 2022. PMID: 35442731 Free PMC article. Review.
-
Optical coherence tomography for observing development of pulmonary arterial vasa vasorum after bidirectional cavopulmonary connection in children.PLoS One. 2019 Apr 8;14(4):e0215146. doi: 10.1371/journal.pone.0215146. eCollection 2019. PLoS One. 2019. PMID: 30958848 Free PMC article. Clinical Trial.
-
Emerging concepts in smooth muscle contributions to airway structure and function: implications for health and disease.Am J Physiol Lung Cell Mol Physiol. 2016 Dec 1;311(6):L1113-L1140. doi: 10.1152/ajplung.00370.2016. Epub 2016 Oct 14. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27742732 Free PMC article. Review.
-
Extracellular purines in lung endothelial permeability and pulmonary diseases.Front Physiol. 2024 Aug 20;15:1450673. doi: 10.3389/fphys.2024.1450673. eCollection 2024. Front Physiol. 2024. PMID: 39234309 Free PMC article. Review.
-
The molecular mechanism responsible for HbSC retinopathy may depend on the action of the angiogenesis-related genes ROBO1 and SLC38A5.Exp Biol Med (Maywood). 2024 Jul 24;249:10070. doi: 10.3389/ebm.2024.10070. eCollection 2024. Exp Biol Med (Maywood). 2024. PMID: 39114443 Free PMC article.
References
-
- Alvarez DF, Huang L, King JA, ElZarrad MK, Yoder MC, Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol 294: L419–L430, 2008 - PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997 - PubMed
-
- Davie NJ, Crossno JT, Jr, Frid MG, Hofmeister SE, Reeves JT, Hyde DM, Carpenter TC, Brunetti JA, McNiece IK, Stenmark KR. Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells. Am J Physiol Lung Cell Mol Physiol 286: L668–L678, 2004 - PubMed
-
- Davie NJ, Gerasimovskaya EV, Hofmeister SE, Richman AP, Jones PL, Reeves JT, Stenmark KR. Pulmonary artery adventitial fibroblasts cooperate with vasa vasorum endothelial cells to regulate vasa vasorum neovascularization: a process mediated by hypoxia and endothelin-1. Am J Pathol 168: 1793–1807, 2006 - PMC - PubMed
-
- Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. FASEB J 17: 984–992, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

