Differential activation of chemically identified neurons in the caudal nucleus of the solitary tract in non-entrained rats after intake of satiating vs. non-satiating meals
- PMID: 24508750
- PMCID: PMC4125568
- DOI: 10.1016/j.physbeh.2014.01.015
Differential activation of chemically identified neurons in the caudal nucleus of the solitary tract in non-entrained rats after intake of satiating vs. non-satiating meals
Abstract
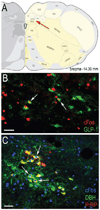
Satiety signals arising from the gastrointestinal (GI) tract and related digestive organs during food ingestion and digestion are conveyed by vagal sensory afferents to the hindbrain nucleus of the solitary tract (NST). Two intermingled but chemically distinct NST neuronal populations have been implicated in meal size control: noradrenergic (NA) neurons that comprise the A2 cell group, and glucagon-like peptide-1 (GLP-1)-positive neurons. Previous results indicate that A2 neurons are activated in a meal size-dependent manner in rats that have been acclimated/entrained to a feeding schedule in order to increase meal size, whereas feeding under the same conditions does not activate GLP-1 neurons. The present study was designed to test the hypothesis that both A2 and GLP-1 neuronal populations are recruited in non-entrained rats after voluntary first-time intake of an unrestricted, satiating volume of liquid Ensure. DBH-positive A2 neurons within the caudal visceral NST were progressively recruited to express cFos in rats that consumed progressively larger volumes of Ensure. Among these DBH-positive neurons, the prolactin-releasing peptide (PrRP)-positive subset was more sensitive to feeding-induced activation than the PrRP-negative subset. Notably, significant activation of GLP-1-positive neurons occurred only in rats that consumed the largest volumes of Ensure, corresponding to nearly 5% of their BW. We interpret these results as evidence that progressive recruitment of NA neurons within the caudal NST, especially the most caudally-situated PrRP-positive subset, effectively "tracks" the magnitude of GI satiety signals and other meal-related sensory feedback. Conversely, GLP-1 neurons may only be recruited in response to the homeostatic challenge of consuming a very large, unanticipated meal.
Keywords: A2 cell group; Glucagon-like peptide-1; Prolactin-releasing peptide; Re-feeding; Satiety; cFos.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Overnight food deprivation markedly attenuates hindbrain noradrenergic, glucagon-like peptide-1, and hypothalamic neural responses to exogenous cholecystokinin in male rats.Physiol Behav. 2013 Sep 10;121:35-42. doi: 10.1016/j.physbeh.2013.01.012. Epub 2013 Feb 4. Physiol Behav. 2013. PMID: 23391574 Free PMC article.
-
Ghrelin signaling contributes to fasting-induced attenuation of hindbrain neural activation and hypophagic responses to systemic cholecystokinin in rats.Am J Physiol Regul Integr Comp Physiol. 2020 May 1;318(5):R1014-R1023. doi: 10.1152/ajpregu.00346.2019. Epub 2020 Apr 15. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 32292065 Free PMC article.
-
Hindbrain glucagon-like peptide-1 neurons track intake volume and contribute to injection stress-induced hypophagia in meal-entrained rats.Am J Physiol Regul Integr Comp Physiol. 2016 May 15;310(10):R906-16. doi: 10.1152/ajpregu.00243.2015. Epub 2016 Mar 2. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 26936779 Free PMC article.
-
The role of nucleus of the solitary tract glucagon-like peptide-1 and prolactin-releasing peptide neurons in stress: anatomy, physiology and cellular interactions.Br J Pharmacol. 2022 Feb;179(4):642-658. doi: 10.1111/bph.15576. Epub 2021 Jun 26. Br J Pharmacol. 2022. PMID: 34050926 Free PMC article. Review.
-
Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure.Brain Res. 2010 Sep 2;1350:18-34. doi: 10.1016/j.brainres.2010.03.059. Epub 2010 Mar 27. Brain Res. 2010. PMID: 20353764 Free PMC article. Review.
Cited by
-
Simplified CLARITY for visualizing immunofluorescence labeling in the developing rat brain.Brain Struct Funct. 2016 May;221(4):2375-83. doi: 10.1007/s00429-015-1020-0. Epub 2015 Mar 14. Brain Struct Funct. 2016. PMID: 25772507 Free PMC article.
-
Genetically and functionally defined NTS to PBN brain circuits mediating anorexia.Nat Commun. 2016 Jun 15;7:11905. doi: 10.1038/ncomms11905. Nat Commun. 2016. PMID: 27301688 Free PMC article.
-
High Fat Diet Attenuates Cholecystokinin-Induced cFos Activation of Prolactin-Releasing Peptide-Expressing A2 Noradrenergic Neurons in the Caudal Nucleus of the Solitary Tract.Neuroscience. 2020 Nov 1;447:113-121. doi: 10.1016/j.neuroscience.2019.08.054. Epub 2019 Sep 10. Neuroscience. 2020. PMID: 31518655 Free PMC article.
-
Eating in mice with gastric bypass surgery causes exaggerated activation of brainstem anorexia circuit.Int J Obes (Lond). 2016 Jun;40(6):921-8. doi: 10.1038/ijo.2016.38. Epub 2016 Mar 17. Int J Obes (Lond). 2016. PMID: 26984418 Free PMC article.
-
Toward a Wiring Diagram Understanding of Appetite Control.Neuron. 2017 Aug 16;95(4):757-778. doi: 10.1016/j.neuron.2017.06.014. Neuron. 2017. PMID: 28817798 Free PMC article. Review.
References
-
- Smith GP. The direct and indirect controls of meal size. Neuroscience and Biobehavioral Reviews. 1996;20:41. - PubMed
-
- Woods SC, Ramsay DS. Pavlovian influences over food and drug intake. Behav Brain Res. 2000 Jun 1;110:175. - PubMed
-
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006 Sep 21;443:289. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

