Memory regulatory T cells reside in human skin
- PMID: 24509084
- PMCID: PMC3934172
- DOI: 10.1172/JCI72932
Memory regulatory T cells reside in human skin
Abstract
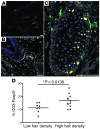
Regulatory T cells (Tregs), which are characterized by expression of the transcription factor Foxp3, are a dynamic and heterogeneous population of cells that control immune responses and prevent autoimmunity. We recently identified a subset of Tregs in murine skin with properties typical of memory cells and defined this population as memory Tregs (mTregs). Due to the importance of these cells in regulating tissue inflammation in mice, we analyzed this cell population in humans and found that almost all Tregs in normal skin had an activated memory phenotype. Compared with mTregs in peripheral blood, cutaneous mTregs had unique cell surface marker expression and cytokine production. In normal human skin, mTregs preferentially localized to hair follicles and were more abundant in skin with high hair density. Sequence comparison of TCRs from conventional memory T helper cells and mTregs isolated from skin revealed little homology between the two cell populations, suggesting that they recognize different antigens. Under steady-state conditions, mTregs were nonmigratory and relatively unresponsive; however, in inflamed skin from psoriasis patients, mTregs expanded, were highly proliferative, and produced low levels of IL-17. Taken together, these results identify a subset of Tregs that stably resides in human skin and suggest that these cells are qualitatively defective in inflammatory skin disease.
Figures





Comment in
-
Regulatory T cells: human skin residency.Nat Rev Immunol. 2014 Mar;14(3):136-7. doi: 10.1038/nri3630. Epub 2014 Feb 14. Nat Rev Immunol. 2014. PMID: 24525839 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- P01 AI035297/AI/NIAID NIH HHS/United States
- U19 AI056388/AI/NIAID NIH HHS/United States
- R01 AI73656/AI/NIAID NIH HHS/United States
- U19 AI56388/AI/NIAID NIH HHS/United States
- R01 AR065174/AR/NIAMS NIH HHS/United States
- K08 AR062064/AR/NIAMS NIH HHS/United States
- K08 AR057763/AR/NIAMS NIH HHS/United States
- P01 AI35297/AI/NIAID NIH HHS/United States
- T32 AI007334/AI/NIAID NIH HHS/United States
- R01AR065174/AR/NIAMS NIH HHS/United States
- 1K08AR062064-01/AR/NIAMS NIH HHS/United States
- K08AR057763/AR/NIAMS NIH HHS/United States
- R01 AI073656/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

