Addressing missing data in clinical studies of kidney diseases
- PMID: 24509298
- PMCID: PMC4078963
- DOI: 10.2215/CJN.10141013
Addressing missing data in clinical studies of kidney diseases
Abstract
Missing data constitute a problem present in all studies of medical research. The most common approach to handling missing data-complete case analysis-relies on assumptions about missing data that rarely hold in practice. The implications of this approach are biased and inefficient descriptions of relationships of interest. Here, various approaches for handling missing data in clinical studies are described. In particular, this work promotes the use of multiple imputation methods that rely on assumptions about missingness that are more flexible than those assumptions relied on by the most common method in use. Furthermore, multiple imputation methods are becoming increasingly more accessible in mainstream statistical software packages, making them both a sound and practical choice. The use of multiple imputation methods is illustrated with examples pertinent to kidney research, and concrete guidance on their use is provided.
Keywords: biostatistics; epidemiology and outcomes; outcomes.
Copyright © 2014 by the American Society of Nephrology.
Figures
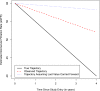

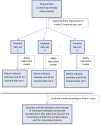
References
-
- US Renal Data System : USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012
-
- Scientific Registry for Transplant Recipients: Available at: http://www.srtr.org Accessed December 17, 2013
-
- Greenland S, Finkle WD: A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol 142: 1255–1264, 1995 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

