Opposing effects of collagen I and vitronectin on fibronectin fibril structure and function
- PMID: 24509439
- PMCID: PMC4019769
- DOI: 10.1016/j.matbio.2014.01.017
Opposing effects of collagen I and vitronectin on fibronectin fibril structure and function
Abstract
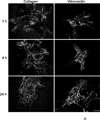
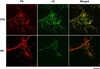
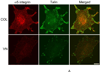
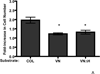
Extracellular matrix fibronectin fibrils serve as passive structural supports for the organization of cells into tissues, yet can also actively stimulate a variety of cell and tissue functions, including cell proliferation. Factors that control and coordinate the functional activities of fibronectin fibrils are not known. Here, we compared effects of cell adhesion to vitronectin versus type I collagen on the assembly of and response to, extracellular matrix fibronectin fibrils. The amount of insoluble fibronectin matrix fibrils assembled by fibronectin-null mouse embryonic fibroblasts adherent to collagen- or vitronectin-coated substrates was not significantly different 20 h after fibronectin addition. However, the fibronectin matrix produced by vitronectin-adherent cells was ~10-fold less effective at enhancing cell proliferation than that of collagen-adherent cells. Increasing insoluble fibronectin levels with the fibronectin fragment, anastellin did not increase cell proliferation. Rather, native fibronectin fibrils polymerized by collagen- and vitronectin-adherent cells exhibited conformational differences in the growth-promoting, III-1 region of fibronectin, with collagen-adherent cells producing fibronectin fibrils in a more extended conformation. Fibronectin matrix assembly on either substrate was mediated by α5β1 integrins. However, on vitronectin-adherent cells, α5β1 integrins functioned in a lower activation state, characterized by reduced 9EG7 binding and decreased talin association. The inhibitory effect of vitronectin on fibronectin-mediated cell proliferation was localized to the cell-binding domain, but was not a general property of αvβ3 integrin-binding substrates. These data suggest that adhesion to vitronectin allows for the uncoupling of fibronectin fibril formation from downstream signaling events by reducing α5β1 integrin activation and fibronectin fibril extension.
Keywords: Cell proliferation; Collagen; Extracellular matrix; Fibronectin; Integrin; Vitronectin.
Copyright © 2014 International Society of Matrix Biology. Published by Elsevier B.V. All rights reserved.
Figures









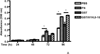
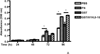







Similar articles
-
Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions.Mol Biol Cell. 2002 Oct;13(10):3546-59. doi: 10.1091/mbc.e02-01-0048. Mol Biol Cell. 2002. PMID: 12388756 Free PMC article.
-
Cell-surface transglutaminase promotes fibronectin assembly via interaction with the gelatin-binding domain of fibronectin: a role in TGFbeta-dependent matrix deposition.J Cell Sci. 2001 Aug;114(Pt 16):2989-3000. doi: 10.1242/jcs.114.16.2989. J Cell Sci. 2001. PMID: 11686302
-
Regional fibronectin and collagen fibril co-assembly directs cell proliferation and microtissue morphology.PLoS One. 2013 Oct 8;8(10):e77316. doi: 10.1371/journal.pone.0077316. eCollection 2013. PLoS One. 2013. PMID: 24116223 Free PMC article.
-
Fibronectin fibrillogenesis, a cell-mediated matrix assembly process.Matrix Biol. 2005 Sep;24(6):389-99. doi: 10.1016/j.matbio.2005.06.008. Matrix Biol. 2005. PMID: 16061370 Review.
-
Shedding New Light on The Role of ανβ3 and α5β1 Integrins in Rheumatoid Arthritis.Molecules. 2019 Apr 18;24(8):1537. doi: 10.3390/molecules24081537. Molecules. 2019. PMID: 31003546 Free PMC article. Review.
Cited by
-
Fibronectin localization and fibrillization are affected by the presence of serum in culture media.Sci Rep. 2015 Mar 23;5:9278. doi: 10.1038/srep09278. Sci Rep. 2015. PMID: 25797118 Free PMC article.
-
Three-dimensional morphogenesis of MDCK cells induced by cellular contractile forces on a viscous substrate.Sci Rep. 2015 Sep 16;5:14208. doi: 10.1038/srep14208. Sci Rep. 2015. PMID: 26374384 Free PMC article.
-
Electrophoretic cytometry of adherent cells.Lab Chip. 2017 Dec 5;17(24):4312-4323. doi: 10.1039/c7lc01012e. Lab Chip. 2017. PMID: 29120467 Free PMC article.
-
Using Acoustic Fields to Fabricate ECM-Based Biomaterials for Regenerative Medicine Applications.Recent Prog Mater. 2020;2(3):10.21926/rpm.2003018. doi: 10.21926/rpm.2003018. Epub 2020 Jul 21. Recent Prog Mater. 2020. PMID: 33604591 Free PMC article.
-
Collagen Type VII (COL7A1) as a Longevity Mediator in Caenorhabditis elegans: Anti-Aging Effects on Healthspan Extension and Skin Collagen Synthesis.Biomol Ther (Seoul). 2024 Nov 1;32(6):801-811. doi: 10.4062/biomolther.2024.127. Epub 2024 Oct 21. Biomol Ther (Seoul). 2024. PMID: 39428402 Free PMC article.
References
-
- Bae E, Sakai T, Mosher DF. Assembly of exogenous fibronectin by fibronectin-null cells is dependent on the adhesive substrate. J Biol Chem. 2004;279:35749–35759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

