HBV life cycle is restricted in mouse hepatocytes expressing human NTCP
- PMID: 24509445
- PMCID: PMC4003384
- DOI: 10.1038/cmi.2013.66
HBV life cycle is restricted in mouse hepatocytes expressing human NTCP
Abstract
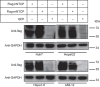
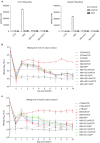
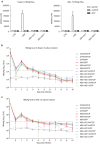
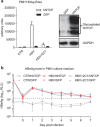
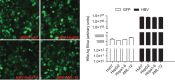
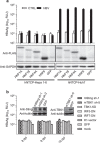
Recent studies have revealed that human sodium taurocholate cotransporting polypeptide (SLC10A1 or NTCP) is a functional cellular receptor for hepatitis B virus (HBV). However, whether human NTCP can support HBV infection in mouse hepatocyte cell lines has not been clarified. Because an HBV-permissible mouse model would be helpful for the study of HBV pathogenesis, it is necessary to investigate whether human NTCP supports the susceptibility of mouse hepatocyte cell lines to HBV. The results show that exogenous human NTCP expression can render non-susceptible HepG2 (human), Huh7 (human), Hepa1-6 (mouse), AML-12 (mouse) cell lines and primary mouse hepatocyte (PMH) cells susceptible to hepatitis D virus (HDV) which employs HBV envelope proteins. However, human NTCP could only introduce HBV susceptibility in human-derived HepG2 and Huh7 cells, but not in mouse-derived Hepa1-6, AML-12 or PMH cells. These data suggest that although human NTCP is a functional receptor that mediates HBV infection in human cells, it cannot support HBV infection in mouse hepatocytes. Our study indicated that the restriction of HBV in mouse hepatocytes likely occurs after viral entry but prior to viral transcription. We have excluded the role of mouse hepatocyte nuclear factors in the restriction of the HBV life cycle and showed that knockdown or inhibition of Sting, TBK1, IRF3 or IRF7, the components of the anti-viral signaling pathways, had no effect on HBV infection in mouse hepatocytes. Therefore, murine restriction factors that limit HBV infection need to be identified before a HBV-permissible mouse line can be created.
Figures
References
-
- Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. - PubMed
-
- Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet. 2011;378:73–85. - PubMed
-
- Gripon P, Le Seyec J, Rumin S, Guguen-Guillouzo C. Myristylation of the hepatitis B virus large surface protein is essential for viral infectivity. Virology. 1995;213:292–299. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

