Loss of Ikaros DNA-binding function confers integrin-dependent survival on pre-B cells and progression to acute lymphoblastic leukemia
- PMID: 24509510
- PMCID: PMC4494688
- DOI: 10.1038/ni.2821
Loss of Ikaros DNA-binding function confers integrin-dependent survival on pre-B cells and progression to acute lymphoblastic leukemia
Abstract
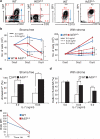
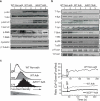
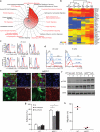
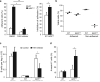
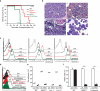
Deletion of the DNA-binding domain of the transcription factor Ikaros generates dominant-negative isoforms that interfere with its activity and correlate with poor prognosis in human precursor B cell acute lymphoblastic leukemia (B-ALL). Here we found that conditional inactivation of the Ikaros DNA-binding domain in early pre-B cells arrested their differentiation at a stage at which integrin-dependent adhesion to niches augmented signaling via mitogen-activated protein kinases, proliferation and self-renewal and attenuated signaling via the pre-B cell signaling complex (pre-BCR) and the differentiation of pre-B cells. Transplantation of polyclonal Ikaros-mutant pre-B cells resulted in long-latency oligoclonal pre-B-ALL, which demonstrates that loss of Ikaros contributes to multistep B cell leukemogenesis. Our results explain how normal pre-B cells transit from a highly proliferative and stroma-dependent phase to a stroma-independent phase during which differentiation is enabled, and suggest potential therapeutic strategies for Ikaros-mutant B-ALL.
Figures


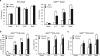
References
-
- Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat Rev Immunol. 2006;6:283–294. - PubMed
-
- Herzog S, Reth M, Jumaa H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat Rev Immunol. 2009;9:195–205. - PubMed
-
- Gauld SB, Cambier JC. Src-family kinases in B-cell development and signaling. Oncogene. 2004;23:8001–8006. - PubMed
-
- Kitamura D, et al. A critical role of lambda 5 protein in B cell development. Cell. 1992;69:823–831. - PubMed
-
- Gong S, Nussenzweig MC. Regulation of an early developmental checkpoint in the B cell pathway by Ig beta. Science. 1996;272:411–414. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

