The integrin β1 subunit regulates paracellular permeability of kidney proximal tubule cells
- PMID: 24509849
- PMCID: PMC3961677
- DOI: 10.1074/jbc.M113.526509
The integrin β1 subunit regulates paracellular permeability of kidney proximal tubule cells
Abstract
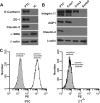
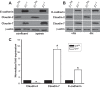
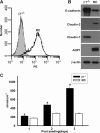
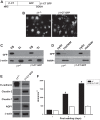
Epithelial cells lining the gastrointestinal tract and kidney have different abilities to facilitate paracellular and transcellular transport of water and solutes. In the kidney, the proximal tubule allows both transcellular and paracellular transport, while the collecting duct primarily facilitates transcellular transport. The claudins and E-cadherin are major structural and functional components regulating paracellular transport. In this study we present the novel finding that the transmembrane matrix receptors, integrins, play a role in regulating paracellular transport of renal proximal tubule cells. Deleting the integrin β1 subunit in these cells converts them from a "loose" epithelium, characterized by low expression of E-cadherin and claudin-7 and high expression of claudin-2, to a "tight" epithelium with increased E-cadherin and claudin-7 expression and decreased claudin-2 expression. This effect is mediated by the integrin β1 cytoplasmic tail and does not entail β1 heterodimerization with an α-subunit or its localization to the cell surface. In addition, we demonstrate that deleting the β1 subunit in the proximal tubule of the kidney results in a major urine-concentrating defect. Thus, the integrin β1 tail plays a key role in regulating the composition and function of tight and adherens junctions that define paracellular transport properties of terminally differentiated renal proximal tubule epithelial cells.
Keywords: Adherens Junction; E-cadherin; Epithelial Cell; Renal Physiology; Tight Junctions.
Figures



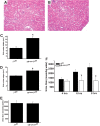
References
-
- Shin K., Fogg V. C., Margolis B. (2006) Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 22, 207–235 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK069921/DK/NIDDK NIH HHS/United States
- R01-DK383069221/DK/NIDDK NIH HHS/United States
- R01 DK095785/DK/NIDDK NIH HHS/United States
- I01 BX002025/BX/BLRD VA/United States
- R01 DK075594/DK/NIDDK NIH HHS/United States
- R01 DK051265/DK/NIDDK NIH HHS/United States
- R01 DK083187/DK/NIDDK NIH HHS/United States
- R01 DK088902/DK/NIDDK NIH HHS/United States
- R01-DK62794/DK/NIDDK NIH HHS/United States
- R01 DK062794/DK/NIDDK NIH HHS/United States
- R01-CA162433/CA/NCI NIH HHS/United States
- R01-DK083187/DK/NIDDK NIH HHS/United States
- R01-DK088902/DK/NIDDK NIH HHS/United States
- R01-DK075594/DK/NIDDK NIH HHS/United States
- I01 BX002196/BX/BLRD VA/United States
- I01 BX000320/BX/BLRD VA/United States
- R01-DK095761/DK/NIDDK NIH HHS/United States
- R01 DK095761/DK/NIDDK NIH HHS/United States
- R01 CA162433/CA/NCI NIH HHS/United States
- R01 -DK95785/DK/NIDDK NIH HHS/United States
- R01-DK51265/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

