Estrogen receptors, the hippocampus, and memory
- PMID: 24510074
- PMCID: PMC4317255
- DOI: 10.1177/1073858413519865
Estrogen receptors, the hippocampus, and memory
Abstract
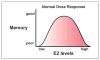
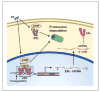
Estradiol effects on memory depend on hormone levels and the interaction of different estrogen receptors within neural circuits. Estradiol induces gene transcription and rapid membrane signaling mediated by estrogen receptor-alpha (ERα), estrogen receptor-beta (ERβ), and a recently characterized G-protein coupled estrogen receptor, each with distinct distributions and ability to influence estradiol-dependent signaling. Investigations using receptor specific agonists suggest that all three receptors rapidly activate kinase-signaling and have complex dose-dependent influences on memory. Research employing receptor knockout mice demonstrate that ERα maintains transcription and memory as estradiol levels decline. This work indicates a regulatory role of ERβ in transcription and cognition, which depends on estradiol levels and the function of ERα. The regulatory role of ERβ is due in part to ERβ acting as a negative regulator of ERα-mediated transcription. Vector-mediated expression of estrogen receptors in the hippocampus provides an innovative research approach and suggests that memory depends on the relative expression of ERα and ERβ interacting with estradiol levels. Notably, the ability of estradiol to improve cognition declines with advanced age along with decreased expression of estrogen receptors. Thus, it will be important for future research to determine the mechanisms that regulate estrogen receptor expression during aging.
Keywords: aging; estrogen; estrogen receptor-alpha (ERα); hippocampus; memory.
© The Author(s) 2014.
Conflict of interest statement
Figures




References
-
- Bake S, Sohrabji F. 17β-Estradiol differentially regulates blood-brain barrier permeability in young and aging female rats. Endocrinology. 2004;145(12):5471–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

