Protein O-mannosylation in metazoan organisms
- PMID: 24510673
- PMCID: PMC3984005
- DOI: 10.1002/0471140864.ps1212s75
Protein O-mannosylation in metazoan organisms
Abstract
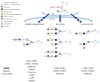
Protein O-mannosylation is a special type of glycosylation that plays prominent roles in metazoans, affecting development and physiology of the nervous system and muscles. A major biological effect of O-mannosylation involves the regulation of α-dystroglycan, a membrane glycoprotein mediating cell-extracellular matrix interactions. Genetic defects of O-mannosylation result in the loss of ligand-binding activity of α-dystroglycan and cause congenital muscular dystrophies termed dystroglycanopathies. Recent progress in mass spectrometry and in vitro analyses has shed new light on the mechanism of α-dystroglycan glycosylation; however, this mechanism is underlain by complex genetic and molecular elements that remain poorly understood. Protein O-mannosylation is evolutionarily conserved in metazoans, yet the pathway is simplified and more amenable to genetic analyses in invertebrate organisms, indicating that genetically tractable in vivo models could facilitate research in this area. This unit describes recent methodological strategies for studying protein O-mannosylation using in vitro and in vivo approaches.
Keywords: O-mannose; Drosophila; O-glycosylation; congenital muscular dystrophy; dystroglycan; mass spectrometry.
Copyright © 2014 John Wiley & Sons, Inc.
Figures




References
-
- Akasaka-Manya K, Manya H, Nakajima A, Kawakita M, Endo T. Physical and functional association of human protein o-mannosyltransferases 1 and 2. J Biol Chem. 2006;281:19339–19345. - PubMed
-
- Anumula KR, Taylor PB. A comprehensive procedure for preparation of partially methylated alditol acetates from glycoprotein carbohydrates. Analytical biochemistry. 1992;203:101–108. - PubMed
-
- Barone R, Aiello C, Race V, Morava E, Foulquier F, Riemersma M, Passarelli C, Concolino D, Carella M, Santorelli F, Vleugels W, Mercuri E, Garozzo D, Sturiale L, Messina S, Jaeken J, Fiumara A, Wevers RA, Bertini E, Matthijs G, Lefeber DJ. DPM2-CDG: A muscular dystrophy-dystroglycanopathy syndrome with severe epilepsy. Ann Neurol. 2012;72:550–558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

