Thrombospondin-1 activation of signal-regulatory protein-α stimulates reactive oxygen species production and promotes renal ischemia reperfusion injury
- PMID: 24511121
- PMCID: PMC4033366
- DOI: 10.1681/ASN.2013040433
Thrombospondin-1 activation of signal-regulatory protein-α stimulates reactive oxygen species production and promotes renal ischemia reperfusion injury
Erratum in
- J Am Soc Nephrol. 2014 Aug;25(8):1884
Abstract
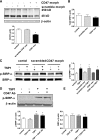
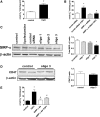
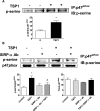
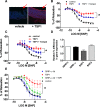
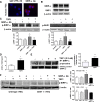
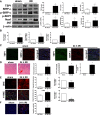
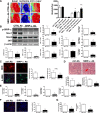
Ischemia reperfusion injury (IRI) causes tissue and organ injury, in part, through alterations in tissue blood flow and the production of reactive oxygen species. The cell surface receptor signal-regulatory protein-α (SIRP-α) is expressed on inflammatory cells and suppresses phagocytosis, but the function of SIRP-α in IRI has not been determined. We reported previously that the matricellular protein thrombospondin-1 is upregulated in IRI. Here, we report a novel interaction between thrombospondin-1 and SIRP-α on nonphagocytic cells. In cell-free experiments, thrombospondin-1 bound SIRP-α. In vascular smooth muscle cells and renal tubular epithelial cells, treatment with thrombospondin-1 led to phosphorylation of SIRP-α and downstream activation of Src homology domain 2-containing phosphatase-1. Thrombospondin-1 also stimulated phosphorylation of p47(phox) (an organizer subunit for nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 1/2) and increased production of superoxide, both of which were abrogated by knockdown or antibody blockade of SIRP-α. In rodent aortic rings, treatment with thrombospondin-1 increased the production of superoxide and inhibited nitric oxide-mediated vasodilation in a SIRP-α-dependent manner. Renal IRI upregulated the thrombospondin-1-SIRP-α signaling axis and was associated with increased superoxide production and cell death. A SIRP-α antibody that blocks thrombospondin-1 activation of SIRP-α mitigated the effects of renal IRI, increasing blood flow, suppressing production of reactive oxygen species, and preserving cellular architecture. A role for CD47 in SIRP-α activation in these pathways is also described. Overall, these results suggest that thrombospondin-1 binding to SIRP-α on nonphagocytic cells activates NADPH oxidase, limits vasodilation, and promotes renal IRI.
Copyright © 2014 by the American Society of Nephrology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

