Comparison of in vitro dialysis release methods of loperamide-encapsulated liposomal gel for topical drug delivery
- PMID: 24511230
- PMCID: PMC3915021
- DOI: 10.2147/IJN.S55805
Comparison of in vitro dialysis release methods of loperamide-encapsulated liposomal gel for topical drug delivery
Abstract
Background: The purpose of this study was to determine the most appropriate dialysis equilibrium method to assess liposomal gel formulations containing hydrophobic drugs, to give the most accurate indication of drug release.
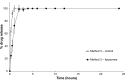
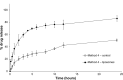
Methods: Loperamide hydrochloride-encapsulated liposomes, composed of L-α-phosphatidylcholine and cholesterol (molar ratio of 2:1), were prepared according to the method of dried lipid film hydration. The liposomes were incorporated into a carbopol gel (0.5%, weight/weight). The release of the drug from the nanoparticles was assessed using a number of variations of the dialysis technique, taking into account solubility parameters and formulation. Method 1 (below saturation point) and Method 2 (above saturation point) used a dilution method to evaluate how drug concentration and solubility affects the in vitro drug-release profile of loperamide hydrochloride, while Methods 3 (below saturation point) and 4 (above saturation point) evaluated how drug concentration and the gel base affect the release profile.
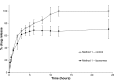
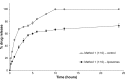
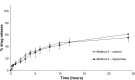
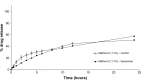
Results: In Method 1, the liposomes showed a rapid release of just over 60% in the first 3 hours and then a slower, sustained release to just over 70% at 24 hours. Method 2 showed a gradual, sustained release profile with the liposomes with 55% release at 24 hours. In Method 3, the liposomes showed a rapid burst release of 98% at 2 hours. In Method 4, the liposomal gel had a rapid release of 60% within 3 hours and then a more gradual, sustained release with 86% release at 24 hours. The free drug suspension in Methods 2 and 4 showed a limited release across the dialysis membrane, in comparison to Methods 1 and 3, which showed a complete release in a timely manner.
Conclusion: This study has demonstrated that the actual method used for equilibrium dialysis plays a significant role in determining the true characteristics of a topical nanoformulation, with Method 3 providing the most accurate indication of the release of a hydrophobic drug from a topical liposomal formulation.
Keywords: gel; in vitro drug release; liposomes; loperamide; topical; transdermal.
Figures
References
-
- Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Del Rev. 2013;65(1):36–48. - PubMed
-
- Pierre MB, Dos Santos Miranda Costa I. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch Dermatol Res. 2011;303(9):607–621. - PubMed
-
- D’Souza SS, DeLuca PP. Methods to assess in vitro drug release from injectable polymeric particulate systems. Pharm Res. 2006;23(3):460–474. - PubMed
-
- Hitzman CJ, Wiedmann TS, Dai H, Elmquist WF. Measurement of drug release from microcarriers by microdialysis. J Pharm Sci. 2005;94(7):1456–1466. - PubMed
-
- Washington C. Drug release from microdisperse systems: a critical review. Int J Pharm. 1990;58(1):1–12.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

