Magnetic field effects in flavoproteins and related systems
- PMID: 24511388
- PMCID: PMC3915827
- DOI: 10.1098/rsfs.2013.0037
Magnetic field effects in flavoproteins and related systems
Abstract
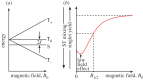
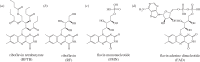
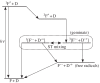
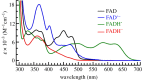
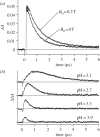
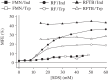
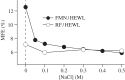
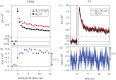
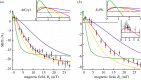
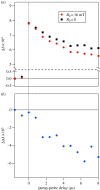
Within the framework of the radical pair mechanism, magnetic fields may alter the rate and yields of chemical reactions involving spin-correlated radical pairs as intermediates. Such effects have been studied in detail in a variety of chemical systems both experimentally and theoretically. In recent years, there has been growing interest in whether such magnetic field effects (MFEs) also occur in biological systems, a question driven most notably by the increasing body of evidence for the involvement of such effects in the magnetic compass sense of animals. The blue-light photoreceptor cryptochrome is placed at the centre of this debate and photoexcitation of its bound flavin cofactor has indeed been shown to result in the formation of radical pairs. Here, we review studies of MFEs on free flavins in model systems as well as in blue-light photoreceptor proteins and discuss the properties that are crucial in determining the magnetosensitivity of these systems.
Keywords: cryptochrome; magnetic compass; magnetic field effect; photolyase; radical pair mechanism.
Figures


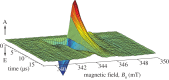
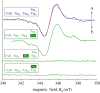
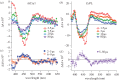

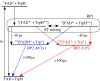
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
