Human cardiosphere-derived cells from advanced heart failure patients exhibit augmented functional potency in myocardial repair
- PMID: 24511463
- PMCID: PMC3914736
- DOI: 10.1016/j.jchf.2013.08.008
Human cardiosphere-derived cells from advanced heart failure patients exhibit augmented functional potency in myocardial repair
Abstract
Objectives: This study sought to compare the regenerative potency of cells derived from healthy and diseased human hearts.
Background: Results from pre-clinical studies and the CADUCEUS (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) trial support the notion that cardiosphere-derived cells (CDCs) from normal and recently infarcted hearts are capable of regenerating healthy heart tissue after myocardial infarction (MI). It is unknown whether CDCs derived from advanced heart failure (HF) patients retain the same regenerative potency.
Methods: In a mouse model of acute MI, we compared the regenerative potential and functional benefits of CDCs derived from 3 groups: 1) non-failing (NF) donor: healthy donor hearts post-transplantation; 2) MI: patients who had an MI 9 to 35 days before biopsy; and 3) HF: advanced cardiomyopathy tissue explanted at cardiac transplantation.
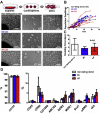
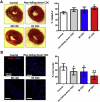
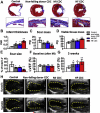
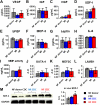
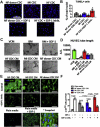
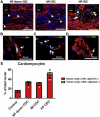
Results: Cell growth and phenotype were identical in all 3 groups. Injection of HF CDCs led to the greatest therapeutic benefit in mice, with the highest left ventricular ejection fraction, thickest infarct wall, most viable tissue, and least scar 3 weeks after treatment. In vitro assays revealed that HF CDCs secreted higher levels of stromal cell-derived factor (SDF)-1, which may contribute to the cells' augmented resistance to oxidative stress, enhanced angiogenesis, and improved myocyte survival. Histological analysis indicated that HF CDCs engrafted better, recruited more endogenous stem cells, and induced greater angiogenesis and cardiomyocyte cell-cycle re-entry. CDC-secreted SDF-1 levels correlated with decreases in scar mass over time in CADUCEUS patients treated with autologous CDCs.
Conclusions: CDCs from advanced HF patients exhibit augmented potency in ameliorating ventricular dysfunction post-MI, possibly through SDF-1–mediated mechanisms.
Figures


Comment in
-
Could cardiosphere-derived cells from patients with heart failure exhibit improved functional repair potential?JACC Heart Fail. 2014 Feb;2(1):62-4. doi: 10.1016/j.jchf.2013.09.007. Epub 2014 Jan 8. JACC Heart Fail. 2014. PMID: 24622119 No abstract available.
References
-
- White AJ, Smith RR, Matsushita S, et al. Intrinsic cardiac origin of human cardiosphere-derived cells. Eur Heart J. 2013;34:68–75. - PubMed
-
- Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

