Potential role of porcine reproductive and respiratory syndrome virus structural protein GP2 in apoptosis inhibition
- PMID: 24511529
- PMCID: PMC3910534
- DOI: 10.1155/2014/160505
Potential role of porcine reproductive and respiratory syndrome virus structural protein GP2 in apoptosis inhibition
Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) is a serious threat to the pork industry, and its pathogenesis needs further investigations. To study the role of two structural proteins of PRRSV in virus-host cells interactions, two stable cell lines (MARC-2a and MARC-N) expressing GP2 and N proteins, respectively, were established. We induced apoptosis in these cells by treating them with staurosporine and found a significant reduction in the number of apoptotic cells in MARC-2a as compared to MARC-N and MARC-145 cells. In addition, we found significantly higher activities of transcriptional factors (NF- κ B and AP-1) in both cell lines as compared to MARC-145 (parent cells). Overall, our data suggest that, although both stable cell lines activate NF- κ B and AP-1, GP2 triggers the antiapoptotic process through an intermediate step that needs to be further investigated.
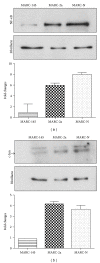
Figures



Similar articles
-
Integrin β3, a RACK1 interacting protein, is critical for porcine reproductive and respiratory syndrome virus infection and NF-κB activation in Marc-145 cells.Virus Res. 2020 Jun;282:197956. doi: 10.1016/j.virusres.2020.197956. Epub 2020 Apr 2. Virus Res. 2020. PMID: 32247758
-
Role of non-structural protein 2 in the regulation of the replication of the porcine reproductive and respiratory syndrome virus in MARC-145 cells: effect of gene silencing and over expression.Vet Microbiol. 2012 Dec 28;161(1-2):58-65. doi: 10.1016/j.vetmic.2012.07.011. Epub 2012 Jul 17. Vet Microbiol. 2012. PMID: 22959006
-
Induction of Apoptosis by the Nonstructural Protein 4 and 10 of Porcine Reproductive and Respiratory Syndrome Virus.PLoS One. 2016 Jun 16;11(6):e0156518. doi: 10.1371/journal.pone.0156518. eCollection 2016. PLoS One. 2016. PMID: 27310256 Free PMC article.
-
Carbon Monoxide Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication by the Cyclic GMP/Protein Kinase G and NF-κB Signaling Pathway.J Virol. 2016 Dec 16;91(1):e01866-16. doi: 10.1128/JVI.01866-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795439 Free PMC article.
-
Apoptosis and porcine reproductive and respiratory syndrome virus.Vet Immunol Immunopathol. 2004 Dec 8;102(3):131-42. doi: 10.1016/j.vetimm.2004.09.004. Vet Immunol Immunopathol. 2004. PMID: 15507300 Review.
Cited by
-
Chitosan-Coated Selenium Nanoparticles Attenuate PRRSV Replication and ROS/JNK-Mediated Apoptosis in vitro.Int J Nanomedicine. 2022 Jul 7;17:3043-3054. doi: 10.2147/IJN.S370585. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35832119 Free PMC article.
-
Analysis of the genetic evolution and recombination of the PRRSV-2 GP2 protein in China from 1996 to 2023.Microbiol Spectr. 2025 Mar 19;13(5):e0307924. doi: 10.1128/spectrum.03079-24. Online ahead of print. Microbiol Spectr. 2025. PMID: 40105345 Free PMC article.
-
Anti-apoptosis in porcine respiratory and reproductive syndrome virus.Virulence. 2016 Jul 3;7(5):610-1. doi: 10.1080/21505594.2016.1162371. Epub 2016 Mar 23. Virulence. 2016. PMID: 27008530 Free PMC article. No abstract available.
-
Modulation of innate immune signaling by nonstructural protein 1 (nsp1) in the family Arteriviridae.Virus Res. 2014 Dec 19;194:100-9. doi: 10.1016/j.virusres.2014.09.007. Epub 2014 Sep 28. Virus Res. 2014. PMID: 25262851 Free PMC article. Review.
-
Activation of regulated cell death in the lung of piglets infected with virulent PRRSV-1 Lena strain occurs earlier and mediated by cleaved Caspase-8.Vet Res. 2021 Jan 22;52(1):12. doi: 10.1186/s13567-020-00882-x. Vet Res. 2021. PMID: 33482914 Free PMC article.
References
-
- Music N, Gagnon CA. The role of porcine reproductive and respiratory syndrome (PRRS) virus structural and non-structural proteins in virus pathogenesis. Animal Health Research Reviews. 2010;11(2):135–163. - PubMed
-
- Snijder EJ, Kikkert M, Fang Y. Arterivirus molecular biology and pathogenesis. The Journal of General Virology. 2013;94(part 10):2141–2163. - PubMed
-
- Miller LC, Fox JM. Apoptosis and porcine reproductive and respiratory syndrome virus. Veterinary Immunology and Immunopathology. 2004;102(3):131–142. - PubMed
-
- Kim TS, Benfield DA, Rowland RRR. Porcine reproductive and respiratory syndrome virus-induced cell death exhibits features consistent with a nontypical form of apoptosis. Virus Research. 2002;85(2):133–140. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

