Mesenchymal stem cells for regenerative therapy: optimization of cell preparation protocols
- PMID: 24511552
- PMCID: PMC3912818
- DOI: 10.1155/2014/951512
Mesenchymal stem cells for regenerative therapy: optimization of cell preparation protocols
Abstract
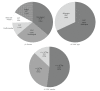
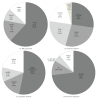
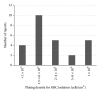
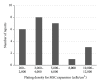
Administration of bone marrow-derived mesenchymal stem cells (MSCs) is an innovative approach for the treatment of a range of diseases that are not curable by current therapies including heart failure. A number of clinical trials have been completed and many others are ongoing; more than 2,000 patients worldwide have been administered with culture-expanded allogeneic or autologous MSCs for the treatment of various diseases, showing feasibility and safety (and some efficacy) of this approach. However, protocols for isolation and expansion of donor MSCs vary widely between these trials, which could affect the efficacy of the therapy. It is therefore important to develop international standards of MSC production, which should be evidence-based, regulatory authority-compliant, of good medical practice grade, cost-effective, and clinically practical, so that this innovative approach becomes an established widely adopted treatment. This review article summarizes protocols to isolate and expand bone marrow-derived MSCs in 47 recent clinical trials of MSC-based therapy, which were published after 2007 onwards and provided sufficient methodological information. Identified issues and possible solutions associated with the MSC production methods, including materials and protocols for isolation and expansion, are discussed with reference to relevant experimental evidence with aim of future clinical success of MSC-based therapy.
Figures
References
-
- Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplantation. 1995;16(4):557–564. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

