The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities
- PMID: 24512032
- PMCID: PMC4159937
- DOI: 10.1089/ars.2013.5378
The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities
Abstract
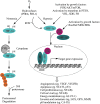
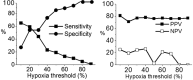
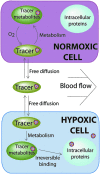
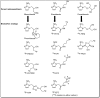
Tumor hypoxia is a well-established biological phenomenon that affects the curability of solid tumors, regardless of treatment modality. Especially for head and neck cancer patients, tumor hypoxia is linked to poor patient outcomes. Given the biological problems associated with tumor hypoxia, the goal for clinicians has been to identify moderately to severely hypoxic tumors for differential treatment strategies. The "gold standard" for detecting and characterizing of tumor hypoxia are the invasive polarographic electrodes. Several less invasive hypoxia assessment techniques have also shown promise for hypoxia assessment. The widespread incorporation of hypoxia information in clinical tumor assessment is severely impeded by several factors, including regulatory hurdles and unclear correlation with potential treatment decisions. There is now an acute need for approved diagnostic technologies for determining the hypoxia status of cancer lesions, as it would enable clinical development of personalized, hypoxia-based therapies, which will ultimately improve outcomes. A number of different techniques for assessing tumor hypoxia have evolved to replace polarographic pO2 measurements for assessing tumor hypoxia. Several of these modalities, either individually or in combination with other imaging techniques, provide functional and physiological information of tumor hypoxia that can significantly improve the course of treatment. The assessment of tumor hypoxia will be valuable to radiation oncologists, surgeons, and biotechnology and pharmaceutical companies who are engaged in developing hypoxia-based therapies or treatment strategies.
Figures






Comment in
-
The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities.Antioxid Redox Signal. 2015 Apr 1;22(10):878-80. doi: 10.1089/ars.2014.6155. Epub 2014 Dec 10. Antioxid Redox Signal. 2015. PMID: 25340660 No abstract available.
-
Author response.Antioxid Redox Signal. 2015 Apr 1;22(10):880. Antioxid Redox Signal. 2015. PMID: 25954780 No abstract available.
References
-
- Adam MF, Gabalski EC, Bloch DA, Oehlert JW, Brown JM, Elsaid AA, Pinto HA, and Terris DJ. Tissue oxygen distribution in head and neck cancer patients. Head Neck 21: 146–153, 1999 - PubMed
-
- Airley RE, Loncaster J, Raleigh JA, Harris AL, Davidson SE, Hunter RD, West CM, and Stratford IJ. GLUT-1 and CAIX as intrinsic markers of hypoxia in carcinoma of the cervix: relationship to pimonidazole binding. Int J Cancer 104: 85–91, 2003 - PubMed
-
- Aquino-Parsons C, Luo C, Vikse CM, and Olive PL. Comparison between the comet assay and the oxygen microelectrode for measurement of tumor hypoxia. Radiother Oncol 51: 179–185, 1999 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

