Reactive oxygen species induce procalcitonin expression in trigeminal ganglia glia
- PMID: 24512072
- PMCID: PMC3947709
- DOI: 10.1111/head.12301
Reactive oxygen species induce procalcitonin expression in trigeminal ganglia glia
Abstract
Objective: To examine calcitonin gene-related peptide (CGRP) gene expression under inflammatory conditions using trigeminal ganglia organ cultures as an experimental system. These cultures have increased proinflammatory signaling that may mimic neurogenic inflammation in the migraine state.
Background: The trigeminal nerve sends peripheral pain signals to the central nervous system during migraine. Understanding the dynamic processes that occur within the trigeminal nerve and ganglion may provide insights into events that contribute to migraine pain. A neuropeptide of particular interest is CGRP, which can be elevated and play a causal role in migraine. However, most studies have overlooked a second splice product of the Calca gene that encodes calcitonin (CT), a peptide hormone involved in calcium homeostasis. Importantly, a precursor form of CT called procalcitonin (proCT) can act as a partial agonist at the CGRP receptor and elevated proCT has recently been reported during migraine.
Methods: We used a trigeminal ganglion whole organ explant model, which has previously been demonstrated to induce pro-inflammatory agents in vitro. Quantitative polymerase chain reaction and immunohistochemistry were used to evaluate changes in messenger ribonucleic acid (mRNA) and protein levels of CGRP and proCT.
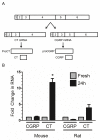
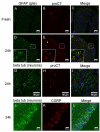
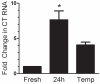
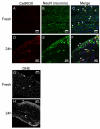
Results: Whole mouse trigeminal ganglia cultured for 24 hours showed a 10-fold increase in CT mRNA, with no change in CGRP mRNA. A similar effect was observed in ganglia from adult rats. ProCT immunoreactivity was localized in glial cells. Cutting the tissue blunted the increase in CT, suggesting that induction required the close environment of the intact ganglia. Consistent with this prediction, there were increased reactive oxygen species in the ganglia, and the elevated CT mRNA was reduced by antioxidant treatment. Surprisingly, reactive oxygen species were increased in neurons, not glia.
Conclusions: These results demonstrate that reactive oxygen species can activate proCT expression from the CGRP gene in trigeminal glia by a paracrine regulatory mechanism. We propose that this glial recruitment pathway may occur following cortical spreading depression and neurogenic inflammation to increase CGRP nociceptive actions in migraine.
Keywords: calcitonin gene-related peptide; migraine; procalcitonin; reactive oxygen species; trigeminal ganglion.
© 2014 American Headache Society.
Figures

References
-
- Ray BS, Wolff HG. Experimental studies on headache - Pain-sensitive structures of the head and their significance in headache. Arch Surg-Chicago. 1940;41:813–856.
-
- Edvinsson L. Tracing neural connections to pain pathways with relevance to primary headaches. Cephalalgia. 2011;31:737–747. - PubMed
-
- Headache Classification Subcommittee of the International Headache S. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. - PubMed
-
- Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8:679–690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

