Polymeric nanoparticles for pulmonary protein and DNA delivery
- PMID: 24512977
- PMCID: PMC4008694
- DOI: 10.1016/j.actbio.2014.01.033
Polymeric nanoparticles for pulmonary protein and DNA delivery
Abstract
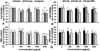
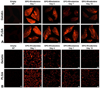
Polymeric nanoparticles (NPs) are promising carriers of biological agents to the lung due to advantages including biocompatibility, ease of surface modification, localized action and reduced systemic toxicity. However, there have been no studies extensively characterizing and comparing the behavior of polymeric NPs for pulmonary protein/DNA delivery both in vitro and in vitro. We screened six polymeric NPs: gelatin, chitosan, alginate, poly(lactic-co-glycolic) acid (PLGA), PLGA-chitosan and PLGA-poly(ethylene glycol) (PEG), for inhalational protein/DNA delivery. All NPs except PLGA-PEG and alginate were <300nm in size with a bi-phasic core compound release profile. Gelatin, PLGA NPs and PLGA-PEG NPs remained stable in deionized water, serum, saline and simulated lung fluid (Gamble's solution) over 5days. PLGA-based NPs and natural polymer NPs exhibited the highest cytocompatibility and dose-dependent in vitro uptake, respectively, by human alveolar type-1 epithelial cells. Based on these profiles, gelatin and PLGA NPs were used to encapsulate plasmid DNA encoding yellow fluorescent protein (YFP) or rhodamine-conjugated erythropoietin (EPO) for inhalational delivery to rats. Following a single inhalation, widespread pulmonary EPO distribution persisted for up to 10days while increasing YFP expression was observed for at least 7days for both NPs. The overall results support both PLGA and gelatin NPs as promising carriers for pulmonary protein/DNA delivery.
Keywords: DNA; Nanoparticles; Nebulization; Protein; Pulmonary.
Copyright © 2014 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures


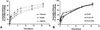

Similar articles
-
Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers.J Control Release. 2012 Jan 10;157(1):149-59. doi: 10.1016/j.jconrel.2011.08.010. Epub 2011 Aug 16. J Control Release. 2012. PMID: 21864595
-
Dry Powder form of Polymeric Nanoparticles for Pulmonary Drug Delivery.Curr Pharm Des. 2016;22(17):2549-60. doi: 10.2174/1381612822666160128150449. Curr Pharm Des. 2016. PMID: 26818872 Review.
-
Low molecular weight chitosan-coated polymeric nanoparticles for sustained and pH-sensitive delivery of paclitaxel.J Drug Target. 2015;23(7-8):725-35. doi: 10.3109/1061186X.2015.1054829. J Drug Target. 2015. PMID: 26453168 Free PMC article.
-
Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: pharmacokinetics and biodistribution profile.Int J Nanomedicine. 2017 Jan 27;12:935-947. doi: 10.2147/IJN.S121881. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28184163 Free PMC article.
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
Cited by
-
Nanostructures for drug delivery in respiratory diseases therapeutics: Revision of current trends and its comparative analysis.J Drug Deliv Sci Technol. 2022 Apr;70:103219. doi: 10.1016/j.jddst.2022.103219. Epub 2022 Mar 5. J Drug Deliv Sci Technol. 2022. PMID: 35280919 Free PMC article. Review.
-
Functional nano-systems for transdermal drug delivery and skin therapy.Nanoscale Adv. 2023 Feb 24;5(6):1527-1558. doi: 10.1039/d2na00530a. eCollection 2023 Mar 14. Nanoscale Adv. 2023. PMID: 36926556 Free PMC article. Review.
-
Self-Propelled Proteomotors with Active Cell-Free mtDNA Clearance for Enhanced Therapy of Sepsis-Associated Acute Lung Injury.Adv Sci (Weinh). 2023 Sep;10(27):e2301635. doi: 10.1002/advs.202301635. Epub 2023 Jul 30. Adv Sci (Weinh). 2023. PMID: 37518854 Free PMC article.
-
The Multifaceted Uses and Therapeutic Advantages of Nanoparticles for Atherosclerosis Research.Materials (Basel). 2018 May 8;11(5):754. doi: 10.3390/ma11050754. Materials (Basel). 2018. PMID: 29738480 Free PMC article. Review.
-
Nanotherapeutics in the treatment of acute respiratory distress syndrome.Life Sci. 2021 Jul 1;276:119428. doi: 10.1016/j.lfs.2021.119428. Epub 2021 Mar 27. Life Sci. 2021. PMID: 33785346 Free PMC article. Review.
References
-
- Andrade F, Videira M, Ferreira D, Sarmento B. Nanocarriers for pulmonary administration of peptides and therapeutic proteins. Nanomedicine. 2011;6:123–141. - PubMed
-
- Beck-Broichsitter M, Merkel OM, Kissel T. Controlled pulmonary drug and gene delivery using polymeric nano-carriers. J Control Release. 2012;161:214–224. - PubMed
-
- Terzano C, Allegra L, Alhaique F, Marianecci C, Carafa M. Non-phospholipid vesicles for pulmonary glucocorticoid delivery. European Journal of Pharmaceutics and Biopharmaceutics. 2005;59:57–62. - PubMed
-
- Dandekar P, Venkataraman C, Mehra A. Pulmonary targeting of nanoparticle drug matrices. J Aerosol Med Pulm Drug Deliv. 2010;23:343–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

