5β-Reduced steroids and human Δ(4)-3-ketosteroid 5β-reductase (AKR1D1)
- PMID: 24513054
- PMCID: PMC3971473
- DOI: 10.1016/j.steroids.2014.01.013
5β-Reduced steroids and human Δ(4)-3-ketosteroid 5β-reductase (AKR1D1)
Abstract
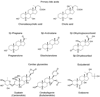
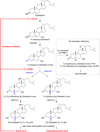
5β-Reduced steroids are non-planar steroids that have a 90° bend in their structure to create an A/B cis-ring junction. This novel property is required for bile-acids to act as emulsifiers, but in addition 5β-reduced steroids have remarkable physiology and may act as potent tocolytic agents, endogenous cardiac glycosides, neurosteroids, and can act as ligands for orphan and membrane bound receptors. In humans there is only a single 5β-reductase gene AKR1D1, which encodes Δ(4)-3-ketosteroid-5β-reductase (AKR1D1). This enzyme is a member of the aldo-keto reductase superfamily, but possesses an altered catalytic tetrad, in which Glu120 replaces the conserved His residue. This predominant liver enzyme generates all 5β-dihydrosteroids in the C19-C27 steroid series. Mutations exist in the AKR1D1 gene, which result in loss of protein stability and are causative in bile-acid deficiency.
Keywords: Bile acid biosynthesis; Enzyme mechanism; Genetics; Steroid metabolism.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



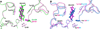

References
-
- Langlois VS, Zhang D, Cooke GM, Trudeau VL. Evolution of steroid-5α-reductases and comparison of their function with 5β-reductase. Gen Comp Endocrinol. 2010;166:489–497. - PubMed
-
- Russell DW, Wilson JD. Steroid 5α-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. - PubMed
-
- Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. - PubMed
-
- Agrawal AA, Petschenka G, Bingham RA, Weber MG, Rasmann S. Toxic cardenolides: chemical ecology and coevolution of specialized plant-herbivore interactions. New Phytol. 2012;194:28–45. - PubMed
-
- Huang X, Warren JT, Gilbert LI. New players in the regulation of ecdysone biosynthesis. J Genet Genomics. 2008;35:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

