Aquaporin and brain diseases
- PMID: 24513456
- PMCID: PMC3960327
- DOI: 10.1016/j.bbagen.2013.10.032
Aquaporin and brain diseases
Abstract
Background: The presence of water channel proteins, aquaporins (AQPs), in the brain led to intense research in understanding the underlying roles of each of them under normal conditions and pathological conditions.
Scope of review: In this review, we summarize some of the recent knowledge on the 3 main AQPs (AQP1, AQP4 and AQP9), with a special focus on AQP4, the most abundant AQP in the central nervous system.
Major conclusions: AQP4 was most studied in several brain pathological conditions ranging from acute brain injuries (stroke, traumatic brain injury) to the chronic brain disease with autoimmune neurodegenerative diseases. To date, no specific therapeutic agents have been developed to either inhibit or enhance water flux through these channels. However, experimental results strongly underline the importance of this topic for future investigation. Early inhibition of water channels may have positive effects in prevention of edema formation in brain injuries but at later time points during the course of a disease, AQP is critical for clearance of water from the brain into blood vessels.
General significance: Thus, AQPs, and in particular AQP4, have important roles both in the formation and resolution of edema after brain injury. The dual, complex function of these water channel proteins makes them an excellent therapeutic target. This article is part of a Special Issue entitled Aquaporins.
Keywords: Blood–brain barrier; Edema; Neuroimaging; Neuroinflammation; Neurovascular unit; Water channel.
© 2013.
Figures
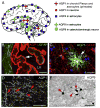
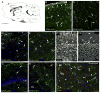
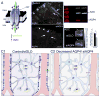


References
-
- Gonen T, Walz T. The structure of aquaporins. Q Rev Biophys. 2006;39:361–396. - PubMed
-
- Badaut J, Lasbennes F, Magistretti PJ, Regli L. Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab. 2002;22:367–378. - PubMed
-
- Badaut J, Nehlig A, Verbavatz J, Stoeckel M, Freund-Mercier MJ, Lasbennes F. Hypervascularization in the magnocellular nuclei of the rat hypothalamus: relationship with the distribution of aquaporin-4 and markers of energy metabolism. J Neuroendocrinol. 2000;12:960–969. - PubMed
-
- Yang M, Gao F, Liu H, Yu WH, Sun SQ. Temporal changes in expression of aquaporin-3, -4, -5 and -8 in rat brains after permanent focal cerebral ischemia. Brain Res. 2009;1290:121–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

