Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems
- PMID: 24514083
- PMCID: PMC4023796
- DOI: 10.1128/AAC.02166-13
Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems
Abstract
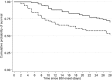
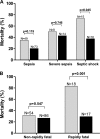
Carbapenemase-producing Klebsiella pneumoniae strains (CP-Kps) are currently among the most important nosocomial pathogens. An observational study was conducted during 2009 to 2010 in two hospitals located in a high-prevalence area (Athens, Greece). The aims were (i) to evaluate the clinical outcome of patients with CP-Kp bloodstream infections (BSIs), (ii) to identify predictors of mortality, and (iii) to evaluate the various antibiotic schemes employed. A total of 205 patients with CP-Kp BSIs were identified: 163 (79.5%) were infected with KPC or KPC and VIM, and 42 were infected with VIM producers. For definitive treatment, 103 patients received combination therapy (two or more active drugs), 72 received monotherapy (one active drug), and 12 received therapy with no active drug. The remaining 18 patients died within 48 h after the onset of bacteremia. The all-cause 28-day mortality was 40%. A significantly higher mortality rate was observed in patients treated with monotherapy than in those treated with combination therapy (44.4% versus 27.2%; P=0.018). The lowest mortality rate (19.3%) was observed in patients treated with carbapenem-containing combinations. In the Cox proportion hazards model, ultimately fatal disease (hazards ratio [HR], 3.25; 95% confidence interval [CI], 1.51 to 7.03; P=0.003), the presence of rapidly fatal underlying diseases (HR, 4.20; 95% CI, 2.19 to 8.08; P<0.001), and septic shock (HR, 2.15; 95% CI, 1.16 to 3.96; P=0.015) were independent predictors of death. Combination therapy was strongly associated with survival (HR of death for monotherapy versus combination, 2.08; 95% CI, 1.23 to 3.51; P=0.006), mostly due to the effectiveness of the carbapenem-containing regimens.
Figures
References
-
- Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 13:785–796. 10.1016/S1473-3099(13)70190-7 - DOI - PMC - PubMed
-
- Livermore DM, Warner M, Mushtaq S, Doumith M, Zhang J, Woodford N. 2011. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int. J. Antimicrob. Agents 37:415–419. 10.1016/j.ijantimicag.2011.01.012 - DOI - PubMed
-
- Chiu SK, Wu TL, Chuang YC, Lin JC, Fung CP, Lu PL, Wang JT, Wang LS, Siu LK, Yeh KM. 2013. National surveillance study on carbapenem non-susceptible Klebsiella pneumoniae in Taiwan: the emergence and rapid dissemination of KPC-2 carbapenemase. PLoS One 8:e69428. 10.1371/journal.pone.0069428 - DOI - PMC - PubMed
-
- Mammina C, Bonura C, Di Bernardo F, Aleo A, Fasciana T, Sodano C, Saporito MA, Verde MS, Tetamo R, Palma DM. 2012. Ongoing spread of colistin-resistant Klebsiella pneumoniae in different wards of an acute general hospital, Italy, June to December 2011 Euro Surveill. 17:20248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

