Degradation of centromeric histone H3 variant Cse4 requires the Fpr3 peptidyl-prolyl Cis-Trans isomerase
- PMID: 24514906
- PMCID: PMC3982699
- DOI: 10.1534/genetics.114.161224
Degradation of centromeric histone H3 variant Cse4 requires the Fpr3 peptidyl-prolyl Cis-Trans isomerase
Abstract
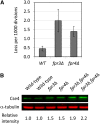
The centromeric histone H3 variant Cse4 in Saccharomyces cerevisiae is polyubiquitylated and degraded in a proteasome-dependent manner. We report here that the proline isomerase Fpr3 regulates Cse4 proteolysis. Structural change in Cse4 by Fpr3 might be important for the interaction between Cse4 and the E3 ubiquitin ligase Psh1.
Keywords: Cse4; Fpr3; Psh1; isomerization; protein degradation.
Figures



References
-
- Arevalo-Rodriguez M., Wu X., Hanes S. D., Heitman J., 2004. Prolyl isomerases in yeast. Front. Biosci. 9: 2420–2446. - PubMed
-
- Collins S. R., Miller K. M., Maas N. L., Roguev A., Fillingham J., et al. , 2007. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature 446: 806–810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

