NO* binds human cystathionine β-synthase quickly and tightly
- PMID: 24515102
- PMCID: PMC3961681
- DOI: 10.1074/jbc.M113.507533
NO* binds human cystathionine β-synthase quickly and tightly
Abstract
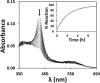
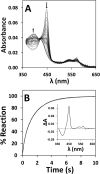
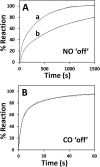
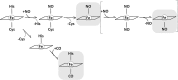
The hexa-coordinate heme in the H2S-generating human enzyme cystathionine β-synthase (CBS) acts as a redox-sensitive regulator that impairs CBS activity upon binding of NO(•) or CO at the reduced iron. Despite the proposed physiological relevance of this inhibitory mechanism, unlike CO, NO(•) was reported to bind at the CBS heme with very low affinity (Kd = 30-281 μm). This discrepancy was herein reconciled by investigating the NO(•) reactivity of recombinant human CBS by static and stopped-flow UV-visible absorption spectroscopy. We found that NO(•) binds tightly to the ferrous CBS heme, with an apparent Kd ≤ 0.23 μm. In line with this result, at 25 °C, NO(•) binds quickly to CBS (k on ∼ 8 × 10(3) m(-1) s(-1)) and dissociates slowly from the enzyme (k off ∼ 0.003 s(-1)). The observed rate constants for NO(•) binding were found to be linearly dependent on [NO(•)] up to ∼ 800 μm NO(•), and >100-fold higher than those measured for CO, indicating that the reaction is not limited by the slow dissociation of Cys-52 from the heme iron, as reported for CO. For the first time the heme of human CBS is reported to bind NO(•) quickly and tightly, providing a mechanistic basis for the in vivo regulation of the enzyme by NO(•). The novel findings reported here shed new light on CBS regulation by NO(•) and its possible (patho)physiological relevance, enforcing the growing evidence for an interplay among the gasotransmitters NO(•), CO, and H2S in cell signaling.
Keywords: Cell Signaling; Enzyme Kinetics; Heme; Hydrogen Sulfide; Redox Regulation.
Figures


References
-
- Li L., Hsu A., Moore P. K. (2009) Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation: a tale of three gases. Pharmacol. Ther. 123, 386–400 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

