Transforming growth factor-β signaling participates in the maintenance of the primordial follicle pool in the mouse ovary
- PMID: 24515103
- PMCID: PMC3961657
- DOI: 10.1074/jbc.M113.532952
Transforming growth factor-β signaling participates in the maintenance of the primordial follicle pool in the mouse ovary
Abstract
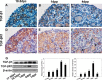
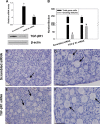
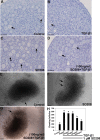
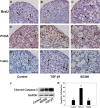
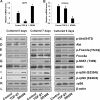
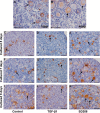
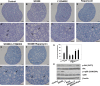
Physiologically, only a few primordial follicles are activated to enter the growing follicle pool each wave. Recent studies in knock-out mice show that early follicular activation depends on signaling from the tuberous sclerosis complex, the mammalian target of rapamycin complex 1 (mTORC1), phosphatase and tensin homolog deleted on chromosome 10, and phosphatidylinositol 3-kinase (PI3K) pathways. However, the manner in which these pathways are normally regulated, and whether or not TGF-β acts on them are poorly understood. So, this study aims to identify whether or not TGF-β acts on the process. Ovary organ culture experiments showed that the culture of 18.5 days post-coitus (dpc) ovaries with TGF-β1 reduced the total population of oocytes and activated follicles, accelerated oocyte growth was observed in ovaries treated with TGF-βR1 inhibitor 2-(5-chloro-2-fluorophenyl)pteridin-4-yl]pyridin-4-yl-amine (SD208) compared with control ovaries, the down-regulation of TGF-βR1 gene expression also activated early primordial follicle oocyte growth. We further showed that there was dramatically more proliferation of granulosa cells in SD208-treated ovaries and less proliferation in TGF-β1-treated ovaries. Western blot and morphological analyses indicated that TGF-β signaling manipulated primordial follicle growth through tuberous sclerosis complex/mTORC1 signaling in oocytes, and the mTORC1-specific inhibitor rapamycin could partially reverse the stimulated effect of SD208 on the oocyte growth and decreased the numbers of growing follicles. In conclusion, our results suggest that TGF-β signaling plays an important physiological role in the maintenance of the dormant pool of primordial follicles, which functions through activation of p70 S6 kinase 1 (S6K1)/ribosomal protein S6 (rpS6) signaling in mouse ovaries.
Keywords: Cell Growth; Mouse; Oocyte; Ovary; Transforming Growth Factor-β (TGFβ).
Figures

References
-
- Pepling M. E. (2006) From primordial germ cell to primordial follicle. Mammalian female germ cell development. Genesis 44, 622–632 - PubMed
-
- Pepling M. E., Spradling A. C. (2001) Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 234, 339–351 - PubMed
-
- Bristol-Gould S. K., Kreeger P. K., Selkirk C. G., Kilen S. M., Cook R. W., Kipp J. L., Shea L. D., Mayo K. E., Woodruff T. K. (2006) Postnatal regulation of germ cells by activin. The establishment of the initial follicle pool. Dev. Biol. 298, 132–148 - PubMed
-
- McGee E. A., Hsueh A. J. (2000) Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 21, 200–214 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

