The 2-oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I
- PMID: 24515115
- PMCID: PMC3961658
- DOI: 10.1074/jbc.M113.545301
The 2-oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I
Abstract
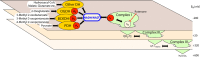
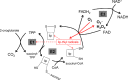
Several flavin-dependent enzymes of the mitochondrial matrix utilize NAD(+) or NADH at about the same operating redox potential as the NADH/NAD(+) pool and comprise the NADH/NAD(+) isopotential enzyme group. Complex I (specifically the flavin, site IF) is often regarded as the major source of matrix superoxide/H2O2 production at this redox potential. However, the 2-oxoglutarate dehydrogenase (OGDH), branched-chain 2-oxoacid dehydrogenase (BCKDH), and pyruvate dehydrogenase (PDH) complexes are also capable of considerable superoxide/H2O2 production. To differentiate the superoxide/H2O2-producing capacities of these different mitochondrial sites in situ, we compared the observed rates of H2O2 production over a range of different NAD(P)H reduction levels in isolated skeletal muscle mitochondria under conditions that favored superoxide/H2O2 production from complex I, the OGDH complex, the BCKDH complex, or the PDH complex. The rates from all four complexes increased at higher NAD(P)H/NAD(P)(+) ratios, although the 2-oxoacid dehydrogenase complexes produced superoxide/H2O2 at high rates only when oxidizing their specific 2-oxoacid substrates and not in the reverse reaction from NADH. At optimal conditions for each system, superoxide/H2O2 was produced by the OGDH complex at about twice the rate from the PDH complex, four times the rate from the BCKDH complex, and eight times the rate from site IF of complex I. Depending on the substrates present, the dominant sites of superoxide/H2O2 production at the level of NADH may be the OGDH and PDH complexes, but these activities may often be misattributed to complex I.
Keywords: 2-Oxoglutarate Dehydrogenase Complex; Branched-chain 2-Oxoacid Dehydrogenase Complex; Branched-chain Ketoacid Dehydrogenase; Mitochondria; NADH Autofluorescence; Pyruvate Dehydrogenase Complex; Rat; Reactive Oxygen Species (ROS); Skeletal Muscle; α-Ketoglutarate Dehydrogenase.
Figures







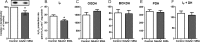

References
-
- Hansford R. G., Hogue B. A., Mildaziene V. (1997) Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J. Bioenerg. Biomembr. 29, 89–95 - PubMed
-
- Barja G., Herrero A. (1998) Localization at complex I and mechanism of the higher free radical production of brain nonsynaptic mitochondria in the short-lived rat than in the longevous pigeon. J. Bioenerg. Biomembr. 30, 235–243 - PubMed
-
- St-Pierre J., Buckingham J. A., Roebuck S. J., Brand M. D. (2002) Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 277, 44784–44790 - PubMed
-
- Andreyev A. Y., Kushnareva Y. E., Starkov A. A. (2005) Mitochondrial metabolism of reactive oxygen species. Biochemistry 70, 200–214 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

